Iridium-Catalyzed Asymmetric Allylic Aromatization Reaction
Dr. Xi-Jia Liu
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorProf. Dr. Chao Zheng
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorYi-Han Yang
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorShicheng Jin
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shu-Li You
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, 300072 China
Search for more papers by this authorDr. Xi-Jia Liu
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorProf. Dr. Chao Zheng
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorYi-Han Yang
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorShicheng Jin
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shu-Li You
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, 300072 China
Search for more papers by this authorGraphical Abstract
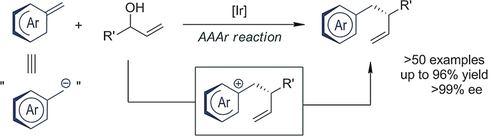
An asymmetric allylic aromatization (AAAr) strategy employs readily accessible equivalents of benzylic nucleophiles in iridium-catalyzed allylic substitution reactions with the concomitant formation of aromatic rings. This strategy provides straightforward access to valuable heteroarenes, bearing a homobenzylic stereogenic center, in enantiopure form.
Abstract
Described herein is an asymmetric allylic aromatization (AAAr) strategy that employs readily accessible equivalents of benzylic nucleophiles in iridium-catalyzed allylic substitution reactions with the concomitant formation of aromatic rings by aromatization. The optimized reaction conditions involving a catalyst derived from a commercially available iridium precursor and the Carreira ligand are compatible with equivalents of benzylic nucleophiles derived from 4- or 5-methyloxazoles, 5-methylthiazoles, 4- or 5-methylfurans, 2- or 3-methylbenzofurans, 3-methylbenzothiophene, 3-methylindole, 1-methylnaphthalene, and methylbenzene. This strategy provides straightforward accesses to valuable heterocyclic aromatic compounds, bearing a homobenzylic stereogenic center, in an enantiopure form and would be difficult to access otherwise. The versatility of the reaction was showcased by the further elaboration of the products into useful building blocks and a drug analogue.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201904156-sup-0001-misc_information.pdf9.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Pharmaceutical Process Development: Current Chemical and Engineering Challenges (Eds.: ), Royal Society of Chemistry, London, 2011;
- 1b Heterocyclic Chemistry in Drug Discovery (Ed.: ), Wiley, Hoboken, 2013;
- 1cE. Vitaku, D. T. Smith, J. T. Njardarson, J. Med. Chem. 2014, 57, 10257–10274.
- 2
- 2a Heterocycles in Natural Product Synthesis (Eds.: ), Wiley-VCH, Weinheim, 2011;
- 2b“Metalation of Azoles and Related Five-Membered Ring Heterocycles”: Topics in Heterocyclic Chemistry, Vol. 29 (Ed.: ), Springer, Heidelberg, 2012;
- 2cR. A. Craig, II, B. M. Stoltz, Chem. Rev. 2017, 117, 7878–7909;
- 2dY. Li, G. Pattenden, Nat. Prod. Rep. 2011, 28, 1269–1310.
- 3
- 3aB. M. Trost, M. L. Crawley, Chem. Rev. 2003, 103, 2921–2944;
- 3bZ. Lu, S. Ma, Angew. Chem. Int. Ed. 2008, 47, 258–297; Angew. Chem. 2008, 120, 264–303;
- 3cJ. D. Weaver, A. Recio, III, A. J. Grenning, J. A. Tunge, Chem. Rev. 2011, 111, 1846–1913; For a book, see:
- 3d“Transition Metal Catalyzed Enantioselective Allylic Substitution”: Organic Synthesis (Ed.: ), Springer, Heidelberg, 2012.
- 4
- 4aB. M. Trost, D. A. Thaisrivongs, J. Am. Chem. Soc. 2008, 130, 14092–14093;
- 4bB. M. Trost, D. A. Thaisrivongs, J. Am. Chem. Soc. 2009, 131, 12056–12057;
- 4cB. M. Trost, D. A. Thaisrivongs, J. Hartwig, J. Am. Chem. Soc. 2011, 133, 12439–12441;
- 4dS.-C. Sha, H. Jiang, J. Mao, A. Bellomo, S. A. Jeong, P. J. Walsh, Angew. Chem. Int. Ed. 2016, 55, 1070–1074; Angew. Chem. 2016, 128, 1082–1086;
- 4eJ. Mao, J. Zhang, H. Jiang, A. Bellomo, M. Zhang, Z. Gao, S. D. Dreher, P. J. Walsh, Angew. Chem. Int. Ed. 2016, 55, 2526–2530; Angew. Chem. 2016, 128, 2572–2576;
- 4fX.-J. Liu, S.-L. You, Angew. Chem. Int. Ed. 2017, 56, 4002–4005; Angew. Chem. 2017, 129, 4060–4063.
- 5R. Murakami, K. Sano, T. Iwai, T. Taniguchi, K. Monde, M. Sawamura, Angew. Chem. Int. Ed. 2018, 57, 9465–9469; Angew. Chem. 2018, 130, 9609–9613.
- 6
- 6aH.-H. Zhang, J.-J. Zhao, S. Yu, J. Am. Chem. Soc. 2018, 140, 16914–16919;
- 6bP. J. Moon, Z. Wei, R. J. Lundgren, J. Am. Chem. Soc. 2018, 140, 17418–17422.
- 7For selected reviews on iridium-catalyzed allylic substitution, see:
- 7aJ. F. Hartwig, L. M. Stanley, Acc. Chem. Res. 2010, 43, 1461–1475;
- 7bW.-B. Liu, J.-B. Xia, S.-L. You, Top. Organomet. Chem. 2012, 38, 155–208;
- 7cJ. Qu, G. Helmchen, Acc. Chem. Res. 2017, 50, 2539–2555;
- 7dQ. Cheng, H.-F. Tu, C. Zheng, J.-P. Qu, G. Helmchen, S.-L. You, Chem. Rev. 2019, 119, 1855–1969; for selected recent Ir-catalyzed asymmetric allylic alkylation reactions, see:
- 7eJ. Y. Hamilton, S. L. Rössler, E. M. Carreira, J. Am. Chem. Soc. 2017, 139, 8082–8085;
- 7fL. Huang, Y. Cai, C. Zheng, L.-X. Dai, S.-L. You, Angew. Chem. Int. Ed. 2017, 56, 10545–10548; Angew. Chem. 2017, 129, 10681–10684;
- 7gT. Sandmeier, S. Krautwald, E. M. Carreira, Angew. Chem. Int. Ed. 2017, 56, 11515–11519; Angew. Chem. 2017, 129, 11673–11677;
- 7hS. E. Shockley, J. C. Hethcox, B. M. Stoltz, Angew. Chem. Int. Ed. 2017, 56, 11545–11548; Angew. Chem. 2017, 129, 11703–11706;
- 7iY.-L. Su, Y.-H. Li, Y.-G. Chen, Z.-Y. Han, Chem. Commun. 2017, 53, 1985–1988;
- 7jX. Jiang, J. J. Beiger, J. F. Hartwig, J. Am. Chem. Soc. 2017, 139, 87–90;
- 7kJ. Chen, X. Zhao, W. Dan, J. Org. Chem. 2017, 82, 10693–10698;
- 7lX. Jiang, P. Boehm, J. F. Hartwig, J. Am. Chem. Soc. 2018, 140, 1239–1242;
- 7mL. Wei, Q. Zhu, S.-M. Xu, X. Chang, C.-J. Wang, J. Am. Chem. Soc. 2018, 140, 1508–1513;
- 7nX. Huo, J. Zhang, J. Fu, R. He, W. Zhang, J. Am. Chem. Soc. 2018, 140, 2080–2084;
- 7oY. Lee, J. Park, S. H. Cho, Angew. Chem. Int. Ed. 2018, 57, 12930–12934; Angew. Chem. 2018, 130, 13112–13116;
- 7pY. Sempere, E. M. Carreira, Angew. Chem. Int. Ed. 2018, 57, 7654–7658; Angew. Chem. 2018, 130, 7780–7784;
- 7qJ. C. Hethcox, S. E. Shockley, B. M. Stoltz, Angew. Chem. Int. Ed. 2018, 57, 8664–8667; Angew. Chem. 2018, 130, 8800–8803;
- 7rR. Sarkar, S. Mitra, S. Mukherjee, Chem. Sci. 2018, 9, 5767–5772;
- 7sT. W. Butcher, J. F. Hartwig, Angew. Chem. Int. Ed. 2018, 57, 13125–13129; Angew. Chem. 2018, 130, 13309–13313;
- 7tC.-Y. Meng, X. Liang, K. Wei, Y.-R. Yang, Org. Lett. 2019, 21, 840;
- 7uS. W. Kim, L. A. Schwartz, J. R. Zbieg, C. E. Stivala, M. J. Krische, J. Am. Chem. Soc. 2019, 141, 671–676.
- 8J. Y. Hamilton, D. Sarlah, E. M. Carreira, J. Am. Chem. Soc. 2014, 136, 3006–3009.
- 9
- 9aG. C. Senadi, W.-P. Hu, J.-S. Hsiao, J. K. Vandavasi, C.-Y. Chen, J.-J. Wang, Org. Lett. 2012, 14, 4478–4481;
- 9bS. Doherty, J. G. Knight, D. O. Perry, N. A. B. Ward, D. M. Bittner, W. McFarlane, C. Wills, M. R. Probert, Organometallics 2016, 35, 1265–1278;
- 9cT. Marcello, L. Testaferri, M. Tingoli, F. Marini, J. Org. Chem. 1993, 58, 1349–1354;
- 9dT. W. Liwosz, S. R. Chemler, Chem. Eur. J. 2013, 19, 12771–12777;
- 9eS. H. Pine, R. Zahler, D. A. Evans, R. H. Grubbs, J. Am. Chem. Soc. 1980, 102, 3270–3272;
- 9fY.-F. Chen, H.-F. Wang, Y. Wang, Y.-C. Luo, H.-L. Zhu, P.-F. Xu, Adv. Synth. Catal. 2010, 352, 1163–1168;
- 9gQ. Tian, J. Bai, B. Chen, G. Zhang, Org. Lett. 2016, 18, 1828–1831;
- 9hY. Zhou, F.-L. Zhu, Z.-T. Liu, X.-M. Zhou, X.-P. Hu, Org. Lett. 2016, 18, 2734–2737;
- 9iP. J. Fricke, J. L. Stasko, D. T. Robbins, A. C. Gardner, J. Stash, M. J. Ferraro, M. W. Fennie, Tetrahedron Lett. 2017, 58, 4510–4513;
- 9jW. Chen, J. Bai, G. Zhang, Adv. Synth. Catal. 2017, 359, 1227–1231;
- 9kM. Bao, H. Nakamura, Y. Yamamoto, J. Am. Chem. Soc. 2001, 123, 759–760;
- 9lB. Peng, S. Zhang, X. Yu, X. Feng, M. Bao, Org. Lett. 2011, 13, 5402–5405;
- 9mS. Zhang, A. Ullah, Y. Yamamoto, M. Bao, Adv. Synth. Catal. 2017, 359, 2723–2728.
- 10
- 10aW. H. Miles, E. A. Dethoff, H. H. Tuson, G. Ulas, J. Org. Chem. 2005, 70, 2862–2865;
- 10bW. Luo, J. Zhao, C. Yin, X. Liu, L. Lin, X. Feng, Chem. Commun. 2014, 50, 7524–7526;
- 10cW. Luo, J. Zhao, J. Ji, L. Lin, X. Liu, H. Mei, X. Feng, Chem. Commun. 2015, 51, 10042–10045;
- 10dB. Wang, Y. Chen, L. Zhou, J. Wang, C.-H. Tung, Z. Xu, J. Org. Chem. 2015, 80, 12718–12724;
- 10eS. Gima, I. Nakamura, M. Terada, Eur. J. Org. Chem. 2017, 4375–4378;
- 10fC. Qu, Z. Wu, W. Li, H. Du, C. Zhu, Adv. Synth. Catal. 2017, 359, 1672–1677;
- 10gW. Liu, W. Cao, H. Hu, L. Lin, X. Feng, Chem. Commun. 2018, 54, 8901–8904;
- 10hK. S. Nalivela, M. Rudolph, E. S. Baeissa, B. G. Alhogbi, I. A. I. Mkhalid, A. S. K. Hashmi, Adv. Synth. Catal. 2018, 360, 2183–2190.
- 11
- 11aM. A. Schafroth, D. Sarlah, S. Krautwald, E. M. Carreira, J. Am. Chem. Soc. 2012, 134, 20276–20278;
- 11bO. F. Jeker, A. G. Kravina, E. M. Carreira, Angew. Chem. Int. Ed. 2013, 52, 12166–12169; Angew. Chem. 2013, 125, 12388–12391;
- 11cM. A. Schafroth, S. M. Rummelt, D. Sarlah, E. M. Carreira, Org. Lett. 2017, 19, 3235–3238.
- 12
- 12aP. A. Evans, D. Uraguchi, J. Am. Chem. Soc. 2003, 125, 7158–7159;
- 12bM. Lafrance, M. Roggen, E. M. Carreira, Angew. Chem. Int. Ed. 2012, 51, 3470–3473; Angew. Chem. 2012, 124, 3527–3530;
- 12cA. T. Meza, T. Wurm, L. Smith, S. W. Kim, J. R. Zbieg, C. E. Stivala, M. J. Krische, J. Am. Chem. Soc. 2018, 140, 1275–1279.
- 13Anon. Amiodarone: Med Lett. Drugs Ther. 1986, 28, 49–50. http://www.ncbi.nlm.nih.gov/pubmed/3702807?dopt=AbstractPlus.
- 14CCDC 1888465 (3 x) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre