Latent Nucleophiles in Lewis Base Catalyzed Enantioselective N-Allylations of N-Heterocycles
You Zi
Institute of Organic Chemistry and Macromolecular Chemistry, Friedrich Schiller University Jena, Humboldtstr. 10, 07743 Jena, Germany
Search for more papers by this authorMarkus Lange
Institute of Organic Chemistry and Macromolecular Chemistry, Friedrich Schiller University Jena, Humboldtstr. 10, 07743 Jena, Germany
Search for more papers by this authorConstanze Schultz
Institute of Organic Chemistry and Macromolecular Chemistry, Friedrich Schiller University Jena, Humboldtstr. 10, 07743 Jena, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Ivan Vilotijevic
Institute of Organic Chemistry and Macromolecular Chemistry, Friedrich Schiller University Jena, Humboldtstr. 10, 07743 Jena, Germany
Search for more papers by this authorYou Zi
Institute of Organic Chemistry and Macromolecular Chemistry, Friedrich Schiller University Jena, Humboldtstr. 10, 07743 Jena, Germany
Search for more papers by this authorMarkus Lange
Institute of Organic Chemistry and Macromolecular Chemistry, Friedrich Schiller University Jena, Humboldtstr. 10, 07743 Jena, Germany
Search for more papers by this authorConstanze Schultz
Institute of Organic Chemistry and Macromolecular Chemistry, Friedrich Schiller University Jena, Humboldtstr. 10, 07743 Jena, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Ivan Vilotijevic
Institute of Organic Chemistry and Macromolecular Chemistry, Friedrich Schiller University Jena, Humboldtstr. 10, 07743 Jena, Germany
Search for more papers by this authorGraphical Abstract
Abstract
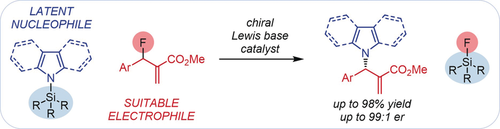
Latent nucleophiles are compounds that are themselves not nucleophilic but can produce a strong nucleophile when activated. Such nucleophiles can expand the scope of Lewis base catalyzed reactions. As a proof of concept, we report that N-silyl pyrroles, indoles, and carbazoles serve as latent N-centered nucleophiles in substitution reactions of allylic fluorides catalyzed by Lewis bases. The reactions feature broad scopes for both reaction partners, excellent regioselectivities, and produce enantioenriched N-allyl pyrroles, indoles, and carbazoles when chiral cinchona alkaloid catalysts are used.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201903392-sup-0001-misc_information.pdf8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. E. Denmark, G. L. Beutner, Angew. Chem. Int. Ed. 2008, 47, 1560–1638; Angew. Chem. 2008, 120, 1584–1663.
- 2
- 2aM. Baidya, G. Y. Remennikov, P. Mayer, H. Mayr, Chem. Eur. J. 2010, 16, 1365–1371;
- 2bG. Zhu, J. Yang, G. Bao, M. Zhang, J. Li, Y. Li, W. Sun, L. Hong, R. Wang, Chem. Commun. 2016, 52, 7882–7885;
- 2cG. Zhan, M.-L. Shi, Q. He, W.-J. Lin, Q. Ouyang, W. Du, Y.-C. Chen, Angew. Chem. Int. Ed. 2016, 55, 2147–2151; Angew. Chem. 2016, 128, 2187–2191.
- 3T.-Y. Liu, M. Xie, Y.-C. Chen, Chem. Soc. Rev. 2012, 41, 4101–4112.
- 4The terms masked and latent nucleophile have been used to refer to a molecule that requires activation to become nucleophilic. For examples, see:
- 4aM. H. Shaw, V. W. Shurtleff, J. A. Terrett, J. D. Cuthbertson, D. W. C. MacMillan, Science 2016, 352, 1304–1308;
- 4bJ. B. Geri, M. M. Wade Wolfe, N. K. Szymczak, J. Am. Chem. Soc. 2018, 140, 9404–9408. We have used the term latent nucleophile to refer to derivatives of otherwise nucleophilic molecules (e.g., indoles) that are not nucleophilic but can be activated (e.g., N-silyl indoles).
- 5
- 5aB. Kempf, N. Hampel, A. R. Ofial, H. Mayr, Chem. Eur. J. 2003, 9, 2209–2218;
- 5bS. Lakhdar, M. Westermaier, F. Terrier, R. Goumont, T. Boubaker, A. R. Ofial, H. Mayr, J. Org. Chem. 2006, 71, 9088–9095;
- 5cT. A. Nigst, M. Westermaier, A. R. Ofial, H. Mayr, Eur. J. Org. Chem. 2008, 2369–2374;
- 5dH. Mayr, S. Lakhdar, B. Maji, A. R. Ofial, Beilstein J. Org. Chem. 2012, 8, 1458–1478.
- 6
- 6aM. Westermaier, H. Mayr, Org. Lett. 2006, 8, 4791–4794;
- 6bC. Schmuck, D. Rupprecht, Synthesis 2007, 3095–3110;
- 6cI. Kumar, R. Kumar, U. Sharma, Synthesis 2018, 50, 2655–2677; for problems with pyrrole nucleophiles in MBH substitutions see: S.-H. Kwon, C.-W. Cho, Bull. Korean Chem. Soc. 2008, 29, 1835–1838; L. Huang, Y. Wei, M. Shi, Org. Biomol. Chem. 2012, 10, 1396–1405.
- 7Despite the high bond dissociation energy of the C−F bond, allylic fluorides have been sucessfully activated in a variety of reactions; see:
- 7aK. Hirano, H. Yorimitsu, K. Oshima, Org. Lett. 2004, 6, 4873–4875;
- 7bL. Hintermann, F. Lang, P. Maire, A. Togni, Eur. J. Inorg. Chem. 2006, 1397–1412;
- 7cJ.-F. Paquin, Synlett 2011, 289–293;
- 7dD. Watanabe, M. Koura, A. Saito, M. Okada, A. Sato, T. Taguchi, J. Fluorine Chem. 2011, 132, 327–338;
- 7eE. Benedetto, M. Keita, M. Tredwell, C. Hollingworth, J. M. Brown, V. Gouverneur, Organometallics 2012, 31, 1408–1416;
- 7fM. Bergeron, D. Guyader, J.-F. Paquin, Org. Lett. 2012, 14, 5888–5891.
- 8For the synthesis of MBH fluorides, see:
- 8aL. Bernardi, B. F. Bonini, M. Comes-Franchini, M. Fochi, M. Folegatti, S. Grilli, A. Mazzanti, A. Ricci, Tetrahedron: Asymmetry 2004, 15, 245–250;
- 8bM. Baumann, I. R. Baxendale, S. V. Ley, Synlett 2008, 2111–2114; for further details on the synthesis of allylic MBH fluorides, see Ref. [14].
- 9
- 9aX. Liu, C. Xu, M. Wang, Q. Liu, Chem. Rev. 2015, 115, 683–730;
- 9bP. Patschinski, C. Zhang, H. Zipse, J. Org. Chem. 2014, 79, 8348–8357;
- 9cM. Marin-Luna, P. Patschinski, H. Zipse, Chem. Eur. J. 2018, 24, 15052–15058;
- 9dM. Marin-Luna, B. Poelloth, F. Zott, H. Zipse, Chem. Sci. 2018, 9, 6509–6515;
- 9eC. P. Johnston, T. H. West, R. E. Dooley, M. Reid, A. B. Jones, E. J. King, A. G. Leach, G. C. Lloyd-Jones, J. Am. Chem. Soc. 2018, 140, 11112–11124.
- 10For recent reviews on modifications of MBH derivatives, see:
- 10aF.-J. Wang, Y. Wei, M. Shi in The Chemistry of the Morita-Baylis-Hillman Reaction, RSC, Cambridge, 2011, pp. 325–484;
- 10bP. Xie, Y. Huang, Org. Biomol. Chem. 2015, 13, 8578–8595;
- 10cN.-J. Zhong, Y.-Z. Wang, L. Cheng, D. Wang, L. Liu, Org. Biomol. Chem. 2018, 16, 5214–5227.
- 11H.-L. Cui, X. Feng, J. Peng, J. Lei, K. Jiang, Y.-C. Chen, Angew. Chem. Int. Ed. 2009, 48, 5737–5740; Angew. Chem. 2009, 121, 5847–5850.
- 12The reactions were qualitatively observed to proceed with relatively high rates up to about 50 % conversion of the fluoride and were then noticeably slower, which is consistent with the dynamic kinetic resolution scenario for these reactions, suggesting that formation of the ammonium intermediate might be the rate-determining step in the reaction mechanism. When the reactions were interrupted before completion, the recovered starting material was enriched in one enantiomer.
- 13J.-P. Jourdan, M. Since, L. El Kihel, C. Lecoutey, S. Corvaisier, R. Legay, J. Sopková-de Oliveira Santos, T. Cresteil, A. Malzert-Fréon, C. Rochais, P. Dallemagne, ChemMedChem 2017, 12, 913–916.
- 14
- 14aY. Du, X. Han, X. Lu, Tetrahedron Lett. 2004, 45, 4967–4971;
- 14bC.-W. Cho, J.-R. Kong, M. J. Krische, Org. Lett. 2004, 6, 1337–1339;
- 14cJ. Wang, H. Li, L.-S. Zu, W. Wang, Org. Lett. 2006, 8, 1391–1394;
- 14dJ. Wang, L. Zu, H. Li, H. Xie, W. Wang, Synthesis 2007, 2576–2580;
- 14eT.-Z. Zhang, L.-X. Dai, X.-L. Hou, Tetrahedron: Asymmetry 2007, 18, 1990–1994;
- 14fG.-N. Ma, S.-H. Cao, M. Shi, Tetrahedron: Asymmetry 2009, 20, 1086–1092;
- 14gS. Gogoi, C.-G. Zhao, D. Ding, Org. Lett. 2009, 11, 2249–2252;
- 14hA. Lin, H. Mao, X. Zhu, H. Ge, R. Tan, C. Zhu, Y. Cheng, Chem. Eur. J. 2011, 17, 13676–13679;
- 14iT. Furukawa, J. Kawazoe, W. Zhang, T. Nishimine, E. Tokunaga, T. Matsumoto, M. Shiro, N. Shibata, Angew. Chem. Int. Ed. 2011, 50, 9684–9688; Angew. Chem. 2011, 123, 9858–9862;
- 14jH. Deng, Y. Wei, M. Shi, Eur. J. Org. Chem. 2011, 1956–1960;
- 14kF. Zhong, J. Luo, G.-Y. Chen, X. Dou, Y. Lu, J. Am. Chem. Soc. 2012, 134, 10222–10227;
- 14lH. Lu, J.-B. Lin, J.-Y. Liu, P.-F. Xu, Chem. Eur. J. 2014, 20, 11659–11663;
- 14mX. Zhao, T. Kang, J. Shen, F. Sha, X. Wu, Chin. J. Chem. 2015, 33, 1333–1337;
- 14nT. T. D. Ngo, T. H. Nguyen, C. Bournaud, R. Guillot, M. Toffano, G. Vo-Thanh, Asian J. Org. Chem. 2016, 5, 895–899;
- 14oM. Kamlar, I. Cisarova, S. Hybelbauerova, J. Vesely, Eur. J. Org. Chem. 2017, 1926–1930;
- 14pH. Wang, L. Yu, M. Xie, J. Wu, G. Qu, K. Ding, H. Guo, Chem. Eur. J. 2018, 24, 1425–1430;
- 14qZ. Li, M. Frings, H. Yu, G. Raabe, C. Bolm, Org. Lett. 2018, 20, 7367–7370;
- 14rH. Wang, C. Guo, Angew. Chem. Int. Ed. 2019, 58, 2854–2858; Angew. Chem. 2019, 131, 2880–2884.
- 15
- 15aT. Furukawa, T. Nishimine, E. Tokunaga, K. Hasegawa, M. Shiro, N. Shibata, Org. Lett. 2011, 13, 3972–3975;
- 15bT. Nishimine, K. Fukushi, N. Shibata, H. Taira, E. Tokunaga, A. Yamano, M. Shiro, N. Shibata, Angew. Chem. Int. Ed. 2014, 53, 517–520; Angew. Chem. 2014, 126, 527–530;
- 15cT. Nishimine, H. Taira, E. Tokunaga, M. Shiro, N. Shibata, Angew. Chem. Int. Ed. 2016, 55, 359–363; Angew. Chem. 2016, 128, 367–371;
- 15dS. Okusu, H. Okazaki, E. Tokunaga, V. A. Soloshonok, N. Shibata, Angew. Chem. Int. Ed. 2016, 55, 6744–6748; Angew. Chem. 2016, 128, 6856–6860;
- 15eT. Nishimine, H. Taira, S. Mori, O. Matsubara, E. Tokunaga, H. Akiyama, V. A. Soloshonok, N. Shibata, Chem. Commun. 2017, 53, 1128–1131. Shibata has pioneered the use of silylated C-nucleophiles in Lewis base catalyzed substitutions of allylic fluorides. Despite the possibility of transferring any of the four carbon substituents from the silicon atom, the least basic substituent is delivered as the nucleophile. We recognized that if a similar concept is applied to N-trialkylsilyl compounds as surrogates of N-centered nucleophiles, only the N-nucleophile would be delivered by virtue of being the least basic substituent on the silicon atom.
- 16T. Stahl, H. F. T. Klare, M. Oestreich, ACS Catal. 2013, 3, 1578–1587.
- 17D. S. Allgäuer, H. Jangra, H. Asahara, Z. Li, Q. Chen, H. Zipse, A. R. Ofial, H. Mayr, J. Am. Chem. Soc. 2017, 139, 13318–13329.