Enantioselective Construction of α-Chiral Silanes by Nickel-Catalyzed C(sp3)−C(sp3) Cross-Coupling
Dr. Hong Yi
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
These authors contributed equally to this work.
Search for more papers by this authorWenbin Mao
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Martin Oestreich
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorDr. Hong Yi
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
These authors contributed equally to this work.
Search for more papers by this authorWenbin Mao
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Martin Oestreich
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorGraphical Abstract
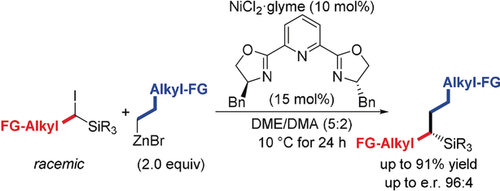
Chiral silicon grease: α-Chiral silanes with no functional group (FG) in direct proximity of the asymmetrically substituted carbon atom are accessible by an enantioselective C(sp3)−C(sp3) cross-coupling of racemic α-silylated alkyl iodides and alkylzinc reagents (see Scheme). The coupling partners can be, but do not need to be, functionalized. α-Chiral silanes can thus be prepared with high modularity.
Abstract
An enantioselective C(sp3)−C(sp3) cross-coupling of racemic α-silylated alkyl iodides and alkylzinc reagents is reported. The reaction is catalyzed by NiCl2/(S,S)-Bn-Pybox and yields α-chiral silanes with high enantiocontrol. The catalyst system does not promote the cross-coupling of the corresponding carbon analogue, corroborating the stabilizing effect of the silyl group on the alkyl radical intermediate (α-silicon effect). Both coupling partners can be, but do not need to be, functionalized, and hence, even α-chiral silanes with no functional group in direct proximity of the asymmetrically substituted carbon atom become accessible. This distinguishes the new method from established approaches for the synthesis of α-chiral silanes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201814340-sup-0001-misc_information.pdf5.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aY. Uozumi, T. Hayashi, J. Am. Chem. Soc. 1991, 113, 9887–9888;
- 1bX. Du, Y. Zhang, D. Peng, Z. Huang, Angew. Chem. Int. Ed. 2016, 55, 6671–6675; Angew. Chem. 2016, 128, 6783–6787;
- 1cM. W. Gribble, Jr., M. T. Pirnot, J. S. Bandar, R. Y. Liu, S. L. Buchwald, J. Am. Chem. Soc. 2017, 139, 2192–2195;
- 1dB. Cheng, P. Lu, H. Zhang, X. Cheng, Z. Lu, J. Am. Chem. Soc. 2017, 139, 9439–9442;
- 1eJ. Chen, B. Cheng, M. Cao, Z. Lu, Angew. Chem. Int. Ed. 2015, 54, 4661–4664; Angew. Chem. 2015, 127, 4744–4747;
- 1fB. Cheng, W. Liu, Z. Lu, J. Am. Chem. Soc. 2018, 140, 5014–5017.
- 2
- 2aC. Walter, G. Auer, M. Oestreich, Angew. Chem. Int. Ed. 2006, 45, 5675–5677; Angew. Chem. 2006, 118, 5803–5805;
- 2bC. Walter, M. Oestreich, Angew. Chem. Int. Ed. 2008, 47, 3818–3820; Angew. Chem. 2008, 120, 3878–3880;
- 2cK.-s. Lee, A. H. Hoveyda, J. Am. Chem. Soc. 2010, 132, 2898–2900;
- 2dJ. M. O'Brien, A. H. Hoveyda, J. Am. Chem. Soc. 2011, 133, 7712–7715.
- 3
- 3aL. B. Delvos, D. J. Vyas, M. Oestreich, Angew. Chem. Int. Ed. 2013, 52, 4650–4653; Angew. Chem. 2013, 125, 4748–4751;
- 3bM. Takeda, R. Shintani, T. Hayashi, J. Org. Chem. 2013, 78, 5007–5017.
- 4T. Lee, J. F. Hartwig, Angew. Chem. Int. Ed. 2016, 55, 8723–8727; Angew. Chem. 2016, 128, 8865–8869.
- 5
- 5aY.-Z. Zhang, S.-F. Zhu, L.-X. Wang, Q.-L. Zhou, Angew. Chem. Int. Ed. 2008, 47, 8496–8498; Angew. Chem. 2008, 120, 8624–8626;
- 5bY. Yasutomi, H. Suematsu, T. Katsuki, J. Am. Chem. Soc. 2010, 132, 4510–4511;
- 5cR. Sambasivan, Z. T. Ball, J. Am. Chem. Soc. 2010, 132, 9289–9291;
- 5dD. Chen, D.-X. Zhu, M.-H. Xu, J. Am. Chem. Soc. 2016, 138, 1498–1501;
- 5eS. B. J. Kan, R. D. Lewis, K. Chen, F. H. Arnold, Science 2016, 354, 1048–1051.
- 6S. Bähr, W. Xue, M. Oestreich, ACS Catal. 2019, 9, 16–24.
- 7
- 7aC. K. Chu, Y. Liang, G. C. Fu, J. Am. Chem. Soc. 2016, 138, 6404–6407;
- 7bW. Xue, Z.-W. Qu, S. Grimme, M. Oestreich, J. Am. Chem. Soc. 2016, 138, 14222–14225;
- 7cW. Xue, R. Shishido, M. Oestreich, Angew. Chem. Int. Ed. 2018, 57, 12141–12145; Angew. Chem. 2018, 130, 12318–12322.
- 8
- 8aA. P. Cinderella, B. Vulovic, D. A. Watson, J. Am. Chem. Soc. 2017, 139, 7741–7744;
- 8bB. Vulovic, A. P. Cinderella, D. A. Watson, ACS Catal. 2017, 7, 8113–8117.
- 9T. Hayashi, M. Konishi, Y. Okamoto, K. Kabeta, M. Kumada, J. Org. Chem. 1986, 51, 3772–3781.
- 10J. L. Hofstra, A. H. Cherney, C. M. Ordner, S. E. Reisman, J. Am. Chem. Soc. 2018, 140, 139–142.
- 11For an overlooked example, see: T. Hayashi, Y. Okamoto, M. Kumada, J. Chem. Soc. Chem. Commun. 1982, 1072–1073.
- 12For authoritative reviews, see:
- 12aG. C. Fu, ACS Cent. Sci. 2017, 3, 692–700;
- 12bJ. Choi, G. C. Fu, Science 2017, 356, eaaf 7230;
- 12cA. Kaga, S. Chiba, ACS Catal. 2017, 7, 4697–4706;
- 12dA. H. Cherney, N. T. Kadunce, S. E. Reisman, Chem. Rev. 2015, 115, 9587–9652.
- 13
- 13aJ. Schmidt, J. Choi, A. T. Liu, M. Slusarczyk, G. C. Fu, Science 2016, 354, 1265–1269; for Fu's earlier work on nickel-catalyzed Negishi cross-couplings to enantioselectively form C(sp3)−C(sp3) bonds, see:
- 13bC. Fischer, G. C. Fu, J. Am. Chem. Soc. 2005, 127, 4594–4595;
- 13cS. Son, G. C. Fu, J. Am. Chem. Soc. 2008, 130, 2756–2757;
- 13dJ. T. Binder, C. J. Cordier, G. C. Fu, J. Am. Chem. Soc. 2012, 134, 17003–17006.
- 14
- 14aA. G. M. Barrett, J. M. Hill, E. M. Wallace, J. Org. Chem. 1992, 57, 386–389;
- 14bA. G. M. Barrett, J. A. Flygare, J. Org. Chem. 1991, 56, 638–642.
- 15H. Nishiyama, H. Sakaguchi, T. Nakamura, M. Horihata, M. Kondo, K. Itoh, Organometallics 1989, 8, 846–848.
- 16K. D. Collins, F. Glorius, Nat. Chem. 2013, 5, 597–601.
- 17M. Chierchia, C. Law, J. P. Morken, Angew. Chem. Int. Ed. 2017, 56, 11870–11874; Angew. Chem. 2017, 129, 12032–12036.
- 18F. C. Whitmore, L. H. Sommer, J. Am. Chem. Soc. 1946, 68, 481–484.