PdII-Catalyzed Regio- and Enantioselective Oxidative C−H/C−H Cross-Coupling Reaction between Ferrocenes and Azoles
Zhong-Jian Cai
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorChen-Xu Liu
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Dr. Qing Gu
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorDr. Chao Zheng
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shu-Li You
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, 300072 China
Search for more papers by this authorZhong-Jian Cai
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorChen-Xu Liu
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Dr. Qing Gu
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorDr. Chao Zheng
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shu-Li You
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, 300072 China
Search for more papers by this authorGraphical Abstract
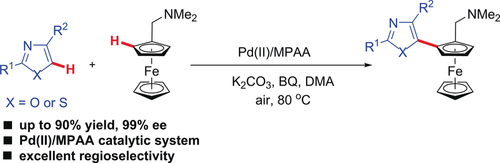
An asymmetric C−H/C−H cross-coupling reaction of ferrocenes with azoles such as oxazoles and thiazoles is presented. Palladium(II)/monoprotected amino acid (MPAA) catalytic system exhibits excellent reactivity and regioselectivity for oxazoles and thiazoles. This method offers a powerful strategy for constructing planar chiral ferrocenes.
Abstract
Asymmetric C−H bond functionalization reaction is one of the most efficient and straightforward methods for the synthesis of optically active molecules. Herein we disclose an asymmetric C−H/C−H cross-coupling reaction of ferrocenes with azoles such as oxazoles and thiazoles. Palladium(II)/monoprotected amino acid (MPAA) catalytic system which exhibits excellent reactivity and regioselectivity for oxazoles and thiazoles. This method offers a powerful strategy for constructing planar chiral ferrocenes. Mechanistic studies suggest that the C−H bond cleavage of azoles is likely proceeding through a SEAr process and may not be a turnover limiting step.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201813887-sup-0001-misc_information.pdf5.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a“C−H Activation”: Topics in Current Chemistry, Vol. 292 (Eds.: ), Springer, Berlin, 2010;
- 1bK. M. Engle, J.-Q. Yu in Organic Chemistry-Breakthroughs and Perspectives (Eds.: ), Wiley-VCH, Weinheim, 2012, pp. 279–323.
10.1002/9783527664801.ch8 Google Scholar
- 2For selected reviews on Pd-catalyzed C−H functionalization, see:
- 2aA. R. Dick, M. S. Sanford, Tetrahedron 2006, 62, 2439;
- 2bD. Alberico, M. E. Scott, M. Lautens, Chem. Rev. 2007, 107, 174;
- 2cC. I. Herrerías, X. Yao, Z. Li, C.-J. Li, Chem. Rev. 2007, 107, 2546;
- 2dT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147;
- 2eC. S. Yeung, V. M. Dong, Chem. Rev. 2011, 111, 1215;
- 2fL. Ackermann, Chem. Rev. 2011, 111, 1315;
- 2gJ. Wencel-Delord, T. Dröge, F. Liu, F. Glorius, Chem. Soc. Rev. 2011, 40, 4740;
- 2hC. Liu, H. Zhang, W. Shi, A. Lei, Chem. Rev. 2011, 111, 1780;
- 2iO. Baudoin, Chem. Soc. Rev. 2011, 40, 4902;
- 2jC. Liu, J. Yuan, M. Gao, S. Tang, W. Li, R. Shi, A. Lei, Chem. Rev. 2015, 115, 12138;
- 2kO. Daugulis, J. Roane, L. D. Tran, Acc. Chem. Res. 2015, 48, 1053;
- 2lG. He, B. Wang, W. A. Nack, G. Chen, Acc. Chem. Res. 2016, 49, 635;
- 2mT. Gensch, M. N. Hopkinson, F. Glorius, J. Wencel-Delord, Chem. Soc. Rev. 2016, 45, 2900;
- 2nO. Baudoin, Acc. Chem. Res. 2017, 50, 1114;
- 2oJ. He, M. Wasa, S. L. K. Chan, Q. Shao, J.-Q. Yu, Chem. Rev. 2017, 117, 8754;
- 2pY. Yang, J. Lan, J. You, Chem. Rev. 2017, 117, 8787.
- 3 Asymmetric Functionalization of C−H Bonds (Ed.: ), RSC, Cambridge, 2015.
- 4For selected reviews, see:
- 4aJ. Wencel-Delord, F. Colobert, Chem. Eur. J. 2013, 19, 14010;
- 4bC. Zheng, S.-L. You, RSC Adv. 2014, 4, 6173;
- 4cC. G. Newton, S.-G. Wang, C. C. Oliveira, N. Cramer, Chem. Rev. 2017, 117, 8908;
- 4dD.-W. Gao, Q. Gu, C. Zheng, S.-L. You, Acc. Chem. Res. 2017, 50, 351;
- 4eT. G. Saint-Denis, R.-Y. Zhu, G. Chen, Q.-F. Wu, J.-Q. Yu, Science 2018, 359, 759.
- 5
- 5aX. Li, J. Yang, M. C. Kozlowski, Org. Lett. 2001, 3, 1137;
- 5bQ.-X. Guo, Z.-J. Wu, Z.-B. Luo, Q.-Z. Liu, J.-L. Ye, S.-W. Luo, L.-F. Cun, L.-Z. Gong, J. Am. Chem. Soc. 2007, 129, 13927;
- 5cH. Egami, T. Katsuki, J. Am. Chem. Soc. 2009, 131, 6082;
- 5dH. Egami, K. Matsumoto, T. Oguma, T. Kunisu, T. Katsuki, J. Am. Chem. Soc. 2010, 132, 13633.
- 6
- 6aD.-W. Gao, Y.-C. Shi, Q. Gu, Z.-L. Zhao, S.-L. You, J. Am. Chem. Soc. 2013, 135, 86;
- 6bD.-W. Gao, Q. Yin, Q. Gu, S.-L. You, J. Am. Chem. Soc. 2014, 136, 4841;
- 6cD.-W. Gao, Q. Gu, S.-L. You, ACS Catal. 2014, 4, 2741;
- 6dJ. Zheng, S.-L. You, Angew. Chem. Int. Ed. 2014, 53, 13244; Angew. Chem. 2014, 126, 13460;
- 6eJ. Zheng, S.-B. Wang, C. Zheng, S.-L. You, J. Am. Chem. Soc. 2015, 137, 4880;
- 6fJ. Zheng, W.-J. Cui, C. Zheng, S.-L. You, J. Am. Chem. Soc. 2016, 138, 5242;
- 6gJ. Zheng, S.-B. Wang, C. Zheng, S.-L. You, Angew. Chem. Int. Ed. 2017, 56, 4540; Angew. Chem. 2017, 129, 4611.
- 7For selected reviews, see:
- 7aL. A. López, E. López, Dalton Trans. 2015, 44, 10128;
- 7bD.-Y. Zhu, P. Chen, J.-B. Xia, ChemCatChem 2016, 8, 68;
- 7cJ.-P. Huang, Q. Gu, S.-L. You, Chin. J. Org. Chem. 2018, 38, 51; for selected examples, see:
- 7dC. Pi, Y. Li, X. Cui, H. Zhang, Y. Han, Y. Wu, Chem. Sci. 2013, 4, 2675;
- 7eC. Pi, X. Cui, X. Liu, M. Guo, H. Zhang, Y. Wu, Org. Lett. 2014, 16, 5164;
- 7fL. Liu, A.-A. Zhang, R.-J. Zhao, F. Li, T.-J. Meng, N. Ishida, M. Murakami, W.-X. Zhao, Org. Lett. 2014, 16, 5336;
- 7gT. Shibata, T. Shizuno, Angew. Chem. Int. Ed. 2014, 53, 5410; Angew. Chem. 2014, 126, 5514;
- 7hR. Deng, Y. Huang, X. Ma, G. Li, R. Zhu, B. Wang, Y. Kang, Z. Gu, J. Am. Chem. Soc. 2014, 136, 4472;
- 7iQ.-W. Zhang, K. An, L.-C. Liu, Y. Yue, W. He, Angew. Chem. Int. Ed. 2015, 54, 6918; Angew. Chem. 2015, 127, 7022;
- 7jS. Zhang, J. Lu, J. Ye, W.-L. Duan, Chin. J. Org. Chem. 2016, 36, 752;
- 7kD. Schmiel, H. Butenschön, Organometallics 2017, 36, 4979;
- 7lS. Luo, Z. Xiong, Y. Lu, Q. Zhu, Org. Lett. 2018, 20, 1837;
- 7mJ. Xu, Y. Liu, J. Zhang, X. Xu, Z. Jin, Chem. Commun. 2018, 54, 689;
- 7nW.-T. Zhao, Z.-Q. Lu, H. Zheng, X.-S. Xue, D. Zhao, ACS Catal. 2018, 8, 7997;
- 7oB.-B. Xu, J. Ye, Y. Yuan, W.-L. Duan, ACS Catal. 2018, 8, 11735;
- 7pW.-J. Kong, Q. Shao, M.-H. Li, Z.-L. Zheng, H. Xu, H.-X. Dai, J.-Q. Yu, Organometallics 2018, 37, 2832.
- 8For oxidative C−H/C−H cross-coupling of ferrocenes with pyrroles, indoles, furans, benzofurans, thiophenes, and benzothiophenes, see: D.-W. Gao, Q. Gu, S.-L. You, J. Am. Chem. Soc. 2016, 138, 2544.
- 9
- 9a“Oxazoles: Synthesis Reactions, and Spectroscopy, Parts A & B” The Chemistry of Heterocyclic Compounds, Vol. 60 (Ed.: ), Wiley, Hoboken, 2004;
- 9bN. Siddiqui, M. F. Arshad, W. Ahsan, M. S. Alam, Int. J. Pharm. Sci. Drug Res. 2009, 1, 136.
- 10 The Agrochemical Handbook (Eds.: ), RSC, University of Nottingham, England, 1991.
- 11
- 11aT. D. Bradshaw, S. Wrigley, D.-F. Shi, R. J. Schultz, K. D. Paull, M. F. G. Stevens, Br. J. Cancer 1998, 77, 745;
- 11bS. Heng, K. R. Gryncel, E. R. Kantrowitz, Bioorg. Med. Chem. 2009, 17, 3916;
- 11cB. R. Copp, Nat. Prod. Rep. 2003, 20, 535;
- 11dN. A. Lack, P. Axerio-Cilies, P. Tavassoli, F. Q. Han, K. H. Chan, C. Feau, E. LeBlanc, E. T. Guns, R. K. Guy, P. S. Rennie, A. Cherkasov, J. Med. Chem. 2011, 54, 8563.
- 12A. Larsson, C. Carlsson, M. Jonsson, B. Albinsson, J. Am. Chem. Soc. 1994, 116, 8459.
- 13
- 13aH. Mochizuki, T. Hasui, M. Kawamoto, T. Shiono, T. Ikeda, C. Adachi, Y. Taniguchi, T. Shirota, Chem. Commun. 2000, 1923;
- 13bA. Mori, A. Sekiguchi, K. Masui, T. Shimada, M. Horie, K. Osakada, M. Kawamoto, T. Ikeda, J. Am. Chem. Soc. 2003, 125, 1700.
- 14
- 14aB. Sezen, D. Sames, Org. Lett. 2003, 5, 3607;
- 14bG. L. Turner, J. A. Morris, M. F. Greaney, Angew. Chem. Int. Ed. 2007, 46, 7996; Angew. Chem. 2007, 119, 8142;
- 14cE. F. Flegeau, M. E. Popkin, M. F. Greaney, Org. Lett. 2008, 10, 2717;
- 14dS. A. Ohnmacht, P. Mamone, A. J. Culshaw, M. F. Greaney, Chem. Commun. 2008, 1241;
- 14eJ. Roger, F. Požgan, H. Doucet, J. Org. Chem. 2009, 74, 1179;
- 14fN. A. Strotman, H. R. Chobanian, Y. Guo, J. He, J. E. Wilson, Org. Lett. 2010, 12, 3578;
- 14gT. Yan, L. Chen, C. Bruneau, P. H. Dixneuf, H. Doucet, J. Org. Chem. 2013, 78, 4177;
- 14hX.-W. Liu, J.-L. Shi, J.-X. Yan, J.-B. Wei, K. Peng, L. Dai, C.-G. Li, B.-Q. Wang, Z.-J. Shi, Org. Lett. 2013, 15, 5774;
- 14iS. Tani, T. N. Uehara, J. Yamaguchi, K. Itami, Chem. Sci. 2014, 5, 123.
- 15For the enantioselective ortho-lithiation of dimethylaminomethylferrocene, see: Y. Nishibayashi, Y. Arikawa, K. Ohe, S. Uemura, J. Org. Chem. 1996, 61, 1172.
- 16For selected examples on Pd/MPAA catalyzed asymmetric C−H bond functionalization, see:
- 16aB.-F. Shi, N. Maugel, Y.-H. Zhang, J.-Q. Yu, Angew. Chem. Int. Ed. 2008, 47, 4882; Angew. Chem. 2008, 120, 4960;
- 16bB.-F. Shi, Y.-H. Zhang, J. K. Lam, D.-H. Wang, J.-Q. Yu, J. Am. Chem. Soc. 2010, 132, 460;
- 16cM. Wasa, K. M. Engle, D. W. Lin, E. J. Yoo, J.-Q. Yu, J. Am. Chem. Soc. 2011, 133, 19598;
- 16dD. G. Musaev, A. Kaledin, B.-F. Shi, J.-Q. Yu, J. Am. Chem. Soc. 2012, 134, 1690;
- 16eL. Chu, X.-C. Wang, C. E. Moore, A. L. Rheingold, J.-Q. Yu, J. Am. Chem. Soc. 2013, 135, 16344;
- 16fX.-F. Cheng, Y. Li, Y.-M. Su, F. Yin, J.-Y. Wang, J. Sheng, H. U. Vora, X.-S. Wang, J.-Q. Yu, J. Am. Chem. Soc. 2013, 135, 1236;
- 16gK.-J. Xiao, D. W. Lin, M. Miura, R.-Y. Zhu, W. Gong, M. Wasa, J.-Q. Yu, J. Am. Chem. Soc. 2014, 136, 8138;
- 16hL. Chu, K.-J. Xiao, J.-Q. Yu, Science 2014, 346, 451;
- 16iK. S. L. Chan, H.-Y. Fu, J.-Q. Yu, J. Am. Chem. Soc. 2015, 137, 2042;
- 16jZ. J. Du, J. Guan, G. J. Wu, P. Xu, L. X. Gao, F. S. Han, J. Am. Chem. Soc. 2015, 137, 632;
- 16kK.-J. Xiao, L. Chu, G. Chen, J.-Q. Yu, J. Am. Chem. Soc. 2016, 138, 7796;
- 16lS.-X. Li, Y.-N. Ma, S.-D. Yang, Org. Lett. 2017, 19, 1842;
- 16mY.-C. Zhu, Y. Li, B.-C. Zhang, F.-X. Zhang, Y.-N. Yang, X.-S. Wang, Angew. Chem. Int. Ed. 2018, 57, 5129; Angew. Chem. 2018, 130, 5223;
- 16nQ.-Y. Sun, W.-Y. Ma, K.-F. Yang, J. Cao, Z.-J. Zheng, Z. Xu, Y.-M. Cui, L.-W. Xu, Chem. Commun. 2018, 54, 10706.
- 17
- 17aG.-J. Cheng, P. Chen, T.-Y. Sun, X. Zhang, J.-Q. Yu, Y.-D. Wu, Chem. Eur. J. 2015, 21, 11180;
- 17bB. E. Haines, J.-Q. Yu, D. G. Musaev, ACS Catal. 2017, 7, 4344.