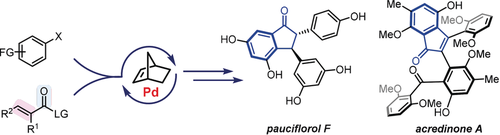
Palladium/Norbornene-Catalyzed Indenone Synthesis from Simple Aryl Iodides: Concise Syntheses of Pauciflorol F and Acredinone A
Feipeng Liu
Department of Chemistry, University of Chicago, Chicago, IL, 60637 USA
Department of Applied Chemistry, China Agricultural University, Beijing, 100193 China
Search for more papers by this authorZhe Dong
Department of Chemistry, University of Chicago, Chicago, IL, 60637 USA
Search for more papers by this authorCorresponding Author
Jianchun Wang
Department of Chemistry, University of Chicago, Chicago, IL, 60637 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Guangbin Dong
Department of Chemistry, University of Chicago, Chicago, IL, 60637 USA
Search for more papers by this authorFeipeng Liu
Department of Chemistry, University of Chicago, Chicago, IL, 60637 USA
Department of Applied Chemistry, China Agricultural University, Beijing, 100193 China
Search for more papers by this authorZhe Dong
Department of Chemistry, University of Chicago, Chicago, IL, 60637 USA
Search for more papers by this authorCorresponding Author
Jianchun Wang
Department of Chemistry, University of Chicago, Chicago, IL, 60637 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Guangbin Dong
Department of Chemistry, University of Chicago, Chicago, IL, 60637 USA
Search for more papers by this authorGraphical Abstract
Abstract
To show the synthetic utility of palladium/norbornene (Pd/NBE) cooperative catalysis, here we report concise syntheses of indenone-based natural products, pauciflorol F and acredinone A, which are enabled by direct annulation between aryl iodides and unsaturated carboxylic acid anhydrides. Compared to the previous indenone-preparation approaches, this method allows simple aryl iodides to be used as substrates with complete control of the regioselectivity. The total synthesis of acredinone A features two different Pd/NBE-catalyzed ortho acylation reactions for constructing penta-substituted arene cores, including the development of a new ortho acylation/ipso borylation.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201813699-sup-0001-misc_information.pdf9.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Morrell, M. Placzek, S. Parmley, B. Grella, S. Antony, Y. Pommier, M. Cushman, J. Med. Chem. 2007, 50, 4388;
- 1bJ. H. Ahn, M. S. Shin, S. H. Jung, S. K. Kang, K. R. Kim, S. D. Rhee, W. H. Jung, S. D. Yang, S. J. Kim, J. R. Woo, J. H. Lee, H. G. Cheon, S. S. Kim, J. Med. Chem. 2006, 49, 4781.
- 2J. L. Jeffrey, R. Sarpong, Org. Lett. 2009, 11, 5450.
- 3
- 3aC. S. Marvel, C. W. Hinman, J. Am. Chem. Soc. 1954, 76, 5435;
- 3bH. Martens, G. Hoornaer, Tetrahedron 1974, 30, 3641;
- 3cA. V. Vasilyev, S. Walspurger, M. Haouas, J. Sommer, P. Pale, A. P. Rudenko, Org. Biomol. Chem. 2004, 2, 3483;
- 3dA. V. Vasilyev, S. Walspurger, P. Pale, J. Sommer, Tetrahedron Lett. 2004, 45, 3379;
- 3eC. Pan, B. Huang, W. Hu, X. Feng, J. Yu, J. Org. Chem. 2016, 81, 2087;
- 3fX.-S. Zhang, J.-Y. Jiao, X.-H. Zhang, B.-L. Hu, X.-G. Zhang, J. Org. Chem. 2016, 81, 5710;
- 3gB. Suchand, G. Satyanarayana, J. Org. Chem. 2017, 82, 372.
- 4
- 4aR. C. Larock, M. J. Doty, S. Cacchi, J. Org. Chem. 1993, 58, 4579;
- 4bD. A. Alonso, C. Najera, M. C. Pacheco, Adv. Synth. Catal. 2002, 344, 172;
- 4cA. A. Pletnev, Q. Tian, R. C. Larock, J. Org. Chem. 2002, 67, 9276;
- 4dT. Miura, M. Murakami, Org. Lett. 2005, 7, 3339;
- 4eY. Harada, J. Nakanishi, H. Fujihara, M. Tobisu, Y. Fukumoto, N. Chatani, J. Am. Chem. Soc. 2007, 129, 5766;
- 4fH. Tsukamoto, Y. Kondo, Org. Lett. 2007, 9, 4227;
- 4gC. -C. Liu, R. P. Korivi, C.-H. Cheng, Chem. Eur. J. 2008, 14, 9503;
- 4hT. Morimoto, K. Yamasaki, A. Hirano, K. Tsutsumi, N. Kagawa, K. Kakiuchi, Y. Harada, Y. Fukumoto, N. Chatani, T. Nishioka, Org. Lett. 2009, 11, 1777;
- 4iX. Yan, S. Zou, P. Zhao, C. Xi, Chem. Commun. 2014, 50, 2775.
- 5
- 5aB.-J. Li, H.-Y. Wang, Q.-L. Zhu, Z.-J. Shi, Angew. Chem. Int. Ed. 2012, 51, 3948; Angew. Chem. 2012, 124, 4014;
- 5bS. Chen, J. Yu, Y. Jiang, F. Chen, J. Cheng, Org. Lett. 2013, 15, 4754;
- 5cZ. Qi, M. Wang, X. Li, Org. Lett. 2013, 15, 5440.
- 6
- 6aM. Catellani, Synlett 2003, 298–313;
- 6bM. Catellani, Top. Organomet. Chem. 2005, 14, 21;
- 6cM. Catellani, E. Motti, N. Della Ca’, Acc. Chem. Res. 2008, 41, 1512;
- 6dA. Martins, B. Mariampillai, M. Lautens, Top. Curr. Chem. 2010, 292, 1;
- 6eJ. Ye, M. Lautens, Nat. Chem. 2015, 7, 863;
- 6fN. Della Ca’, M. Fontana, E. Motti, M. Catellani, Acc. Chem. Res. 2016, 49, 1389.
- 7M. Catellani, F. Frignani, A. Rangoni, Angew. Chem. Int. Ed. Engl. 1997, 36, 119; Angew. Chem. 1997, 109, 142.
- 8For selected examples of Pd/NBE catalysis, see:
- 8aM. Lautens, S. Piguel, Angew. Chem. Int. Ed. 2000, 39, 1045;
10.1002/(SICI)1521-3773(20000317)39:6<1045::AID-ANIE1045>3.0.CO;2-Q CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 1087;
- 8bM. Catellani, E. Motti, S. Baratta, Org. Lett. 2001, 3, 3611;
- 8cF. Faccini, E. Motti, M. Catellani, J. Am. Chem. Soc. 2004, 126, 78;
- 8dC. Bressy, D. Alberico, M. Lautens, J. Am. Chem. Soc. 2005, 127, 13148;
- 8eB. Mariampillai, D. Alberico, V. Bidau, M. Lautens, J. Am. Chem. Soc. 2006, 128, 14436;
- 8fG. Maestri, E. Motti, N. Della Ca’, M. Malacria, E. Derat, M. Catellani, J. Am. Chem. Soc. 2011, 133, 8574;
- 8gZ. Dong, G. Dong, J. Am. Chem. Soc. 2013, 135, 18350;
- 8hH. Zhang, P. Chen, G. Liu, Angew. Chem. Int. Ed. 2014, 53, 10174; Angew. Chem. 2014, 126, 10338;
- 8iZ. Dong, J. Wang, Z. Ren, G. Dong, Angew. Chem. Int. Ed. 2015, 54, 12664; Angew. Chem. 2015, 127, 12855;
- 8jY. Huang, R. Zhu, K. Zhao, Z. Gu, Angew. Chem. Int. Ed. 2015, 54, 12669; Angew. Chem. 2015, 127, 12860;
- 8kP.-X. Zhou, Y.-Y. Ye, C. Liu, L.-B. Zhao, J.-Y. Hou, D.-Q. Chen, Q. Tang, A.-Q. Wang, J.-Y. Zhang, Q.-X. Huang, P.-F. Xu, Y.-M. Liang, ACS Catal. 2015, 5, 4927;
- 8lJ. Wang, L. Zhang, Z. Dong, G. Dong, Chem 2016, 1, 581;
- 8mH.-G. Cheng, C. Wu, H. Chen, R. Chen, G. Qian, Z. Geng, Q. Wei, Y. Xia, J. Zhang, Y. Zhang, Q. Zhou, Angew. Chem. Int. Ed. 2018, 57, 3444; Angew. Chem. 2018, 130, 3502;
- 8nR. Li, G. Dong, Angew. Chem. Int. Ed. 2018, 57, 1697; Angew. Chem. 2018, 130, 1713; for PdII-initiated Pd/NBE catalysis, see:
- 8oL. Jiao, T. Bach, J. Am. Chem. Soc. 2011, 133, 12990;
- 8pL. Jiao, E. Herdtweck, T. Bach, J. Am. Chem. Soc. 2012, 134, 14563;
- 8qX.-C. Wang, W. Gong, L.-Z. Fang, R.-Y. Zhu, S. H. Li, K. M. Engle, J.-Q. Yu, Nature 2015, 519, 334;
- 8rZ. Dong, J. Wang, G. Dong, J. Am. Chem. Soc. 2015, 137, 5887;
- 8sP.-X. Shen, X.-C. Wang, P. Wang, R. Y. Zhu, J.-Q. Yu, J. Am. Chem. Soc. 2015, 137, 11574;
- 8tH. Shi, A. N. Herron, Y. Shao, Q. Shao, J.-Q. Yu, Nature 2018, 558, 581;
- 8uH. Zhang, H.-Y. Wang, Y. Luo, C. Chen, Y. Cao, P. Chen, Y.-L. Guo, Y. Lan, G. Liu, ACS Catal. 2018, 8, 2173.
- 9
- 9aFor the seminal use of bifunctional reagent in Pd/NBE catalysis, see ref. [8a];
- 9bfor the fluorenone synthesis by Pd/NBE catalysis, see: Y.-B. Zhao, B. Mariampillai, D. A. Candito, B. Laleu, M. Li, M. Lautens, Angew. Chem. Int. Ed. 2009, 48, 1849; Angew. Chem. 2009, 121, 1881.
- 10
- 10aX. Sui, R. Zhu, G. Li, X. Ma, Z. Gu, J. Am. Chem. Soc. 2013, 135, 9318;
- 10bH. Weinstabl, M. Suhartono, Z. Qureshi, M. Lautens, Angew. Chem. Int. Ed. 2013, 52, 5305; Angew. Chem. 2013, 125, 5413;
- 10cZ.-S. Liu, G. Qian, Q. Gao, P. Wang, H.-G. Cheng, Q. Wei, Q. Liu, Q. Zhou, ACS Catal. 2018, 8, 4783.
- 11M. Catellani, G. P. Chiusoli, J. Organomet. Chem. 1988, 346, C 27.
- 12The anhydride derived from unsubstituted acrylic acid did not yield the desired product, owing to its instability under the reaction conditions.
- 13
- 13aZ. Dong, G. Lu, J. Wang, P. Liu, G. Dong, J. Am. Chem. Soc. 2018, 140, 8551;
- 13bN. Della Ca, A. Casnati, M. Fontana, G. Coruzzi, B. M. Aresta, N. Corriero, R. Maggi, G. Maestri, E. Motti, ChemCatChem 2018, 10, 4346;
- 13cM. S. A. Elsayed, B. Griggs, M. Cushman, Org. Lett. 2018, 20, 5228.
- 14
- 14aM. Catellani, M. C. Fagnola, Angew. Chem. Int. Ed. Engl. 1994, 33, 2421; Angew. Chem. 1994, 106, 2559;
- 14bJ. Wang, R. Li, Z. Dong, P. Liu, G. Dong, Nat. Chem. 2018, 10, 866.
- 15T. Ito, T. Tanaka, M. Iinuma, K. Nakaya, Y. Takahashi, R. Sawa, J. Murata, D. Darnaedi, J. Nat. Prod. 2004, 67, 932.
- 16
- 16aS. A. Snyder, A. L. Zografos, Y. Lin, Angew. Chem. Int. Ed. 2007, 46, 8186; Angew. Chem. 2007, 119, 8334;
- 16bB. H. Lee, Y. L. Choi, S. Shin, J. N. Heo, J. Org. Chem. 2011, 76, 6611;
- 16cY. Yang, D. Philips, S. Pan, J. Org. Chem. 2011, 76, 1902;
- 16dD. J. Kerr, M. Miletic, N. Manchala, J. M. White, B. L. Flynn, Org. Lett. 2013, 15, 4118.
- 17C.-F. Xiao, Y. Zou, J.-L. Du, H.-Y. Sun, X.-K. Liu, Synth. Commun. 2012, 42, 1243.
- 18CCDC 1881705 (11) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre
- 19H. Kim, I. Yang, S. Y. Ryu, D. H. Won, A. G. Giri, W. Wang, H. Choi, J. Chin, D. Hahn, E. Kim, C. Han, J. Lee, S. J. Nam, W. K. Ho, H. Kang, J. Nat. Prod. 2015, 78, 363.
- 20H. Wulff, N. A. Castle, L. A. Pardo, Nat. Rev. Drug Discovery 2009, 8, 982.
- 21H. Shi, D. J. Babinski, T. Ritter, J. Am. Chem. Soc. 2015, 137, 3775.
- 22T. J. Colacot, H. A. Shea, Org. Lett. 2004, 6, 3731.
- 23G. A. Molander, C. R. Bernardi, J. Org. Chem. 2002, 67, 8424.