Palladium(II)-Catalyzed Enantioselective Aminotrifluoromethoxylation of Unactivated Alkenes using CsOCF3 as a Trifluoromethoxide Source
Dr. Chaohuang Chen
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorPhilipp Miro Pflüger
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraße 40, Münster, 48149 Germany
Search for more papers by this authorDr. Pinhong Chen
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Guosheng Liu
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorDr. Chaohuang Chen
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorPhilipp Miro Pflüger
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraße 40, Münster, 48149 Germany
Search for more papers by this authorDr. Pinhong Chen
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Guosheng Liu
State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorDedicated to Professor Qingyun Chen on the occasion of his 90th birthday
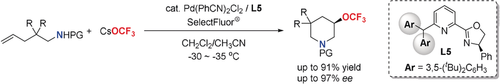
Graphical Abstract
PdII-catalyzed enantioselective intramolecular aminotrifluoromethoxylation of unactivated alkenes using readily accessible and stable CsOCF3 as a trifluoromethoxide source has been developed, which affords a wide variety of enantiomerically enriched β-substituted OCF3-containing piperidines in good yields. Introducing a sterically bulky group into pyridine-oxazoline (Pyox) ligands is crucial to increasing both reactivity and enantioselectivity.
Abstract
Asymmetric PdII-catalyzed intramolecular aminotrifluoromethoxylation of unactivated alkenes using readily accessible and stable CsOCF3 as a trifluoromethoxide source has been developed, which affords a wide variety of enantiomerically enriched β-substituted OCF3-containing piperidines in good yields. Introducing a sterically bulky group into pyridine-oxazoline (Pyox) ligands is crucial to increasing both reactivity and enantioselectivity for the reaction. Additionally, the reaction features good functional group compatibility and mild reaction conditions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201813591-sup-0001-misc_information.pdf16.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok, H. Liu, Chem. Rev. 2014, 114, 2432;
- 1bE. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly, N. A. Meanwell, J. Med. Chem. 2015, 58, 8315;
- 1cC. Ni, J. Hu, Chem. Soc. Rev. 2016, 45, 5441;
- 1dN. A. Meanwell, J. Med. Chem. 2018, 61, 5822.
- 2
- 2aM. G. Campbell, T. Ritter, Chem. Rev. 2015, 115, 612;
- 2bX. Liu, C. Xu, M. Wang, Q. Liu, Chem. Rev. 2015, 115, 683;
- 2cS. Preshlock, M. Tredwell, V. Gouverneur, Chem. Rev. 2016, 116, 719.
- 3For reviews on asymmetric fluorinations, see:
- 3aJ.-A. Ma, D. Cahard, Chem. Rev. 2004, 104, 6119;
- 3bJ.-A. Ma, D. Cahard, Chem. Rev. 2008, 108, PR 1;
- 3cC. Alonso, E. M. Marigorta, G. Rbiales, F. Palacios, Chem. Rev. 2015, 115, 1847;
- 3dY. Yang, T. Wu, R. J. Phipps, F. D. Toste, Chem. Rev. 2015, 115, 826;
- 3eC. Chen, L. Fu, P. Chen, G. Liu, Chin. J. Chem. 2017, 35, 1781.
- 4
- 4aW. Huang, X. Wan, Q. Shen, Angew. Chem. Int. Ed. 2017, 56, 11986; Angew. Chem. 2017, 129, 12148;
- 4bH. Kondo, M. Maeno, K. Hirano, N. Shibata, Chem. Commun. 2018, 54, 5522.
- 5
- 5aC. Huang, T. Liang, S. Harada, E. Lee, T. Ritter, J. Am. Chem. Soc. 2011, 133, 13308;
- 5bC. Zhang, D. A. Vicic, Organometallics 2012, 31, 7812;
- 5cS. Chen, Y. Huang, X. Fang, H. Li, Z. Zhang, T. S. A. Hor, Z. Weng, Dalton Trans. 2015, 44, 19682.
- 6For recent reviews on trifluoromethoxylation, see:
- 6aT. Besset, P. Jubault, X. Pannecoucke, T. Possion, Org. Chem. Front. 2016, 3, 1004;
- 6bA. Tlili, F. Toulgoat, T. Billard, Angew. Chem. Int. Ed. 2016, 55, 11726; Angew. Chem. 2016, 128, 11900; For some recent examples, see:
- 6cO. Marrec, T. Billard, J.-P. Vors, S. Pazenok, B. R. Langlois, J. Fluorine Chem. 2010, 131, 200;
- 6dO. Marrec, T. Billard, J.-P. Vors, S. Pazenok, B. R. Langlois, Adv. Synth. Catal. 2010, 352, 2831;
- 6eK. N. Hojczyk, P. Feng, C. Zhan, M.-Y. Ngai, Angew. Chem. Int. Ed. 2014, 53, 14559; Angew. Chem. 2014, 126, 14787;
- 6fM. Zhou, C. Ni, Y. Zeng, J. Hu, J. Am. Chem. Soc. 2018, 140, 6801;
- 6gW. Zheng, C. A. Moroles-Rivera, J. W. Lee, P. Liu, M.-Y. Ngai, Angew. Chem. Int. Ed. 2018, 57, 9645; Angew. Chem. 2018, 130, 9793;
- 6hB. J. Jelier, P. F. Tripet, E. Pietrasiak, I. Franzoni, G. Jeschke, A. Togni, Angew. Chem. Int. Ed. 2018, 57, 13784; Angew. Chem. 2018, 130, 13980;
- 6iW. Zheng, J. W. Lee, C. A. Morales-Rivera, P. Liu, M.-Y. Ngai, Angew. Chem. Int. Ed. 2018, 57, 13795; Angew. Chem. 2018, 130, 13991.
- 7S. Guo, F. Cong, R. Guo, L. Wang, P. Tang, Nat. Chem. 2017, 9, 546.
- 8For some reviews, see ref. [2], and
- 8aV. V. Grushin, Acc. Chem. Res. 2010, 43, 160;
- 8bC. Hollingworth, V. Gouverneur, Chem. Commun. 2012, 48, 2929;
- 8cP. Chen, G. Liu, Synthesis 2013, 45, 2919;
- 8dX. Mu, G. Liu, Org. Chem. Front. 2014, 1, 430.
- 9N. A. Cochrane, H. Nguyen, M. R. Gagné, J. Am. Chem. Soc. 2013, 135, 628.
- 10
- 10aE. P. A. Talbot, T. A. Fernandes, J. M. McKenna, F. D. Toste, J. Am. Chem. Soc. 2014, 136, 4101;
- 10bY. He, Z. Yang, R. T. Thornbury, F. D. Toste, J. Am. Chem. Soc. 2015, 137, 12207.
- 11H. Park, P. Verma, K. Hong, J.-Q. Yu, Nat. Chem. 2018, 10, 755.
- 12The reductive elimination on the high-valent metal center for a secondaryl C−heteroatom bond formation, generally undergoes a stereo-retention pathway. For details, see refs. [3d,e], and G. Yin, X. Mu, G. Liu, Acc. Chem. Res. 2016, 49, 2413.
- 13
- 13aC. Chen, P. Chen, G. Liu, J. Am. Chem. Soc. 2015, 137, 15648;
- 13bC. Chen, Y. Luo, L. Fu, P. Chen, Y. Lan, G. Liu, J. Am. Chem. Soc. 2018, 140, 1207.
- 14X. Qi, P. Chen, G. Liu, Angew. Chem. Int. Ed. 2017, 56, 9517; Angew. Chem. 2017, 129, 9645.
- 15X. Qi, C. Chen, C. Hou, L. Fu, P. Chen, G. Liu, J. Am. Chem. Soc. 2018, 140, 7415.
- 16For the PyN−Pd bond length (Å): (L2)PdCl2 (2.027(7)) ≪ (L3)PdCl2 (2.131(2)) < (L4)PdCl2 (2.135(12)) ≪ (L1)PdCl2 (2.162(8)). For details, see SI.
- 17Unfortunately, we failed to obtain a crystal of the (L5)PdCl2 complex.
- 18CCDC 1884988 (2 c) and 1884993 (15 a) contain the supplementary crystallographic data. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 19M. Morgenthaler, E. Schweizer, A. Hoffmann-Roder, F. Benini, R. E. Martin, G. Jaeschke, B. Wagner, H. Fischer, S. Bendels, D. Zimmerli, J. Schneider, F. Diederich, M. Kansy, K. Muller, ChemMedChem 2007, 2, 1100. and ref. [1b].
- 20 The Merck index an encyclopedia of chemicals, drugs, and biologicals, 11th ed. ), Merck, Rahway, NJ, 1989, p. 7747.
- 21The deactivation of palladium catalyst might be attributed to the coordination of 30 produced from SelectFluor. In fact, adding external 30 (20 mol %) could remarkably inhibit the reaction under the conditions C (Figure 1); however, the palladium catalyst poisoning was not observed in the presence of L5 (conditions E) or excess amounts of AgOCF3 (conditions A). For details, see the Supporting Information.
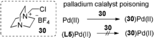
- 22The stereochemistry of the reaction is quite similar to our previous studies (refs. [13a, 15]), see the Supporting Information for the details.