Enantioselective Synthesis of 3,3′-Diaryl-SPINOLs: Rhodium-Catalyzed Asymmetric Arylation/BF3-Promoted Spirocyclization Sequence
Long Yin
Department of Chemistry and State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, 211198 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Junhao Xing
Department of Chemistry and State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, 211198 China
These authors contributed equally to this work.
Search for more papers by this authorYuhan Wang
Department of Chemistry and State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, 211198 China
Search for more papers by this authorYue Shen
Department of Chemistry and State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, 211198 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Tao Lu
Department of Chemistry and State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, 211198 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Tamio Hayashi
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaowei Dou
Department of Chemistry and State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, 211198 China
Search for more papers by this authorLong Yin
Department of Chemistry and State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, 211198 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Junhao Xing
Department of Chemistry and State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, 211198 China
These authors contributed equally to this work.
Search for more papers by this authorYuhan Wang
Department of Chemistry and State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, 211198 China
Search for more papers by this authorYue Shen
Department of Chemistry and State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, 211198 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Tao Lu
Department of Chemistry and State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, 211198 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Tamio Hayashi
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaowei Dou
Department of Chemistry and State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, 211198 China
Search for more papers by this authorGraphical Abstract
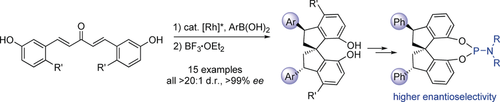
Going for a ′SPIN′: The enantioselective synthesis of a series of 3,3′-diarylated 1,1′-spirobiindane-7,7′-diols (3,3′-diaryl-SPINOLs) was developed using a sequential Rh-catalyzed twofold asymmetric conjugate arylation/BF3-promoted diastereoselective spirocyclization. Some phosphoramidite ligands were prepared from the 3,3′-Ph-SPINOL and applied to several catalytic asymmetric reactions, and the 3,3′-diarylated ligands showed higher enantioselectivities than the privileged nonsubstituted ligands.
Abstract
The enantioselective synthesis of a series of C2-symmetric 3,3′-diarylated 1,1′-spirobiindane-7,7′-diols (3,3′-diaryl-SPINOLs) was developed by sequential Rh-catalyzed twofold asymmetric conjugate arylation/BF3-promoted diastereoselective spirocyclization (>20:1 d.r. and >99 % ee for all examples). Some phosphoramidite ligands were prepared from the 3,3′-Ph-SPINOL and applied to several catalytic asymmetric reactions, and the 3,3′-diarylated ligands showed higher enantioselectivities than the privileged nonsubstituted ligands.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201812266-sup-0001-misc_information.pdf5.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. P. Yoon, E. N. Jacobsen, Science 2003, 299, 1691–1693;
- 1b Privileged Chiral Ligands and Catalysts (Ed.: ), Wiley-VCH, Weiheim, 2011.
- 2V. B. Birman, A. L. Rheingold, K.-C. Lam, Tetrahedron: Asymmetry 1999, 10, 125–131.
- 3For the first application of SPINOL in asymmetric catalysis, see: Y. Fu, J.-H. Xie, A.-G. Hu, H. Zhou, L.-X. Wang, Q.-L. Zhou, Chem. Commun. 2002, 480–481.
- 4For reviews, see:
- 4aJ.-H. Xie, Q.-L. Zhou, Acc. Chem. Res. 2008, 41, 581–593;
- 4bK. Ding, Z. Han, Z. Wang, Chem. Asian J. 2009, 4, 32–41;
- 4cG. B. Bajracharya, M. A. Arai, P. S. Koranne, T. Suzuki, S. Takizawa, H. Sasai, Bull. Chem. Soc. Jpn. 2009, 82, 285–302;
- 4dS.-F. Zhu, Q.-L. Zhou in Privileged Chiral Ligands and Catalysts (Ed.: ), Wiley-VCH, Weiheim, 2011, pp. 137–170;
10.1002/9783527635207.ch4 Google Scholar
- 4eS.-F. Zhu, Q.-L. Zhou, Acc. Chem. Res. 2012, 45, 1365–1377;
- 4fJ.-H. Xie, Q.-L. Zhou, Acta Chim. Sin. 2014, 72, 778–797;
- 4gS.-F. Zhu, Q.-L. Zhou in Ligand Design in Metal Chemistry: Reactivity and Catalysis (Eds.: ), Wiley, Hoboken, 2016, pp. 66–103;
10.1002/9781118839621.ch4 Google Scholar
- 4hY. Liu, W. Li, J. Zhang, Natl. Sci. Rev. 2017, 4, 326–358;
- 4iA. Rahman, X. Lin, Org. Biomol. Chem. 2018, 16, 4753–4777.
- 5For development of SPINOL-based ligands, see:
- 5aA.-G. Hu, Y. Fu, J.-H. Xie, H. Zhou, L.-X. Wang, Q.-L. Zhou, Angew. Chem. Int. Ed. 2002, 41, 2348–2350;
10.1002/1521-3773(20020703)41:13<2348::AID-ANIE2348>3.0.CO;2-K CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 2454–2456;
- 5bJ.-H. Xie, L.-X. Wang, Y. Fu, S.-F. Zhu, B.-M. Fan, H.-F. Duan, Q.-L. Zhou, J. Am. Chem. Soc. 2003, 125, 4404–4405;
- 5cS.-F. Zhu, Y. Fu, J.-H. Xie, B. Liu, L. Xing, Q.-L. Zhou, Tetrahedron: Asymmetry 2003, 14, 3219–3224;
- 5dS.-F. Zhu, Y. Yang, L.-X. Wang, B. Liu, Q.-L. Zhou, Org. Lett. 2005, 7, 2333–2335;
- 5eS.-F. Zhu, J.-B. Xie, Y.-Z. Zhang, S. Li, Q.-L. Zhou, J. Am. Chem. Soc. 2006, 128, 12886–12891;
- 5fB. Liu, S.-F. Zhu, L.-X. Wang, Q.-L. Zhou, Tetrahedron: Asymmetry 2006, 17, 634–641;
- 5gC. Chen, S.-F. Zhu, X.-Y. Wu, Q.-L. Zhou, Tetrahedron: Asymmetry 2006, 17, 2761–2767;
- 5hJ.-B. Xie, J.-H. Xie, X.-Y. Liu, W.-L. Kong, S. Li, Q.-L. Zhou, J. Am. Chem. Soc. 2010, 132, 4538–4539;
- 5iJ.-H. Xie, X.-Y. Liu, J.-B. Xie, L.-X. Wang, Q.-L. Zhou, Angew. Chem. Int. Ed. 2011, 50, 7329–7332; Angew. Chem. 2011, 123, 7467–7470;
- 5jS.-F. Zhu, Y.-B. Yu, S. Li, L.-X. Wang, Q.-L. Zhou, Angew. Chem. Int. Ed. 2012, 51, 8872–8875; Angew. Chem. 2012, 124, 9002–9005;
- 5kJ. Zheng, W.-J. Cui, C. Zheng, S.-L. You, J. Am. Chem. Soc. 2016, 138, 5242–5245; SPINOL-based organocatalysts, see:
- 5lY. K. Chung, G. C. Fu, Angew. Chem. Int. Ed. 2009, 48, 2225–2227; Angew. Chem. 2009, 121, 2259–2261;
- 5mI. Čorić, S. Müller, B. List, J. Am. Chem. Soc. 2010, 132, 17370–17373;
- 5nF. Xu, D. Huang, C. Han, W. Shen, X. Lin, Y. Wang, J. Org. Chem. 2010, 75, 8677–8680;
- 5oC.-H. Xing, Y.-X. Liao, J. Ng, Q.-S. Hu, J. Org. Chem. 2011, 76, 4125–4131;
- 5pB. Xu, S.-F. Zhu, X.-L. Xie, J.-J. Shen, Q.-L. Zhou, Angew. Chem. Int. Ed. 2011, 50, 11483–11486; Angew. Chem. 2011, 123, 11685–11688;
- 5qS. Takizawa, K. Kiriyama, K. Ieki, H. Sasai, Chem. Commun. 2011, 47, 9227–9229; SPINOL-based chiral reagents, see:
- 5rT. Dohi, A. Maruyama, N. Takenaga, K. Senami, Y. Minamitsuji, H. Fujioka, S. B. Caemmerer, Y. Kita, Angew. Chem. Int. Ed. 2008, 47, 3787–3790; Angew. Chem. 2008, 120, 3847–3850;
- 5sT. Dohi, N. Takenaga, T. Nakae, Y. Toyoda, M. Yamasaki, M. Shiro, H. Fujioka, A. Maruyama, Y. Kita, J. Am. Chem. Soc. 2013, 135, 4558–4566.
- 6For selected examples, see:
- 6aB.-M. Fan, J.-H. Xie, S. Li, L.-X. Wang, Q.-L. Zhou, Angew. Chem. Int. Ed. 2007, 46, 1275–1277; Angew. Chem. 2007, 119, 1297–1299;
- 6bB. Liu, S.-F. Zhu, W. Zhang, C. Chen, Q.-L. Zhou, J. Am. Chem. Soc. 2007, 129, 5834–5835;
- 6cY. Yang, S.-F. Zhu, C.-Y. Zhou, Q.-L. Zhou, J. Am. Chem. Soc. 2008, 130, 14052–14053;
- 6dA. Z. González, D. Benitez, E. Tkatchouk, W. A. Goddard, F. D. Toste, J. Am. Chem. Soc. 2011, 133, 5500–5507;
- 6eM. von Delius, C. M. Le, V. M. Dong, J. Am. Chem. Soc. 2012, 134, 15022–15032;
- 6fA. Martínez, M. J. Webber, S. Müller, B. List, Angew. Chem. Int. Ed. 2013, 52, 9486–9490; Angew. Chem. 2013, 125, 9664–9668;
- 6gS.-G. Wang, S.-L. You, Angew. Chem. Int. Ed. 2014, 53, 2194–2197; Angew. Chem. 2014, 126, 2226–2229;
- 6hB. Xu, M.-L. Li, X.-D. Zuo, S.-F. Zhu, Q.-L. Zhou, J. Am. Chem. Soc. 2015, 137, 8700–8703;
- 6iJ.-S. Lin, P. Yu, L. Huang, P. Zhang, B. Tan, X.-Y. Liu, Angew. Chem. Int. Ed. 2015, 54, 7847–7851; Angew. Chem. 2015, 127, 7958–7962;
- 6jW. Yang, J. Sun, Angew. Chem. Int. Ed. 2016, 55, 1868–1871; Angew. Chem. 2016, 128, 1900–1903;
- 6kZ.-L. Tao, A. Adili, H.-C. Shen, Z.-Y. Han, L.-Z. Gong, Angew. Chem. Int. Ed. 2016, 55, 4322–4326; Angew. Chem. 2016, 128, 4394–4398;
- 6lC. Wu, G. Yue, C. D.-T. Nielsen, K. Xu, H. Hirao, J. Zhou, J. Am. Chem. Soc. 2016, 138, 742–745;
- 6mQ. M. Kainz, C. D. Matier, A. Bartoszewicz, S. L. Zultanski, J. C. Peters, G. C. Fu, Science 2016, 351, 681–684;
- 6nS.-G. Wang, Z.-L. Xia, R.-Q. Xu, X.-J. Liu, C. Zheng, S.-L. You, Angew. Chem. Int. Ed. 2017, 56, 7440–7443; Angew. Chem. 2017, 129, 7548–7551;
- 6oL. Zhang, J. Zhang, J. Ma, D.-J. Cheng, B. Tan, J. Am. Chem. Soc. 2017, 139, 1714–1717;
- 6pJ. Li, M. Kong, B. Qiao, R. Lee, X. Zhao, Z. Jiang, Nat. Commun. 2018, 9, 2445–2453;
- 6qJ. Zhang, P. Yu, S.-Y. Li, H. Sun, S.-H. Xiang, J. Wang, K. N. Houk, B. Tan, Science 2018, 361, eaas 8707.
- 7
- 7aJ.-H. Zhang, J. Liao, X. Cui, K.-B. Yu, J. Zhu, J.-G. Deng, S.-F. Zhu, L.-X. Wang, Q.-L. Zhou, L. W. Chung, T. Ye, Tetrahedron: Asymmetry 2002, 13, 1363–1366;
- 7bZ. Li, X. Liang, F. Wu, B. Wan, Tetrahedron: Asymmetry 2004, 15, 665–669;
- 7cZ. Li, X. Liang, B. Wan, F. Wu, Synthesis 2004, 17, 2805–2808;
- 7dS. Lu, S. B. Poh, Y. Zhao, Angew. Chem. Int. Ed. 2014, 53, 11041–11045; Angew. Chem. 2014, 126, 11221–11225.
- 8For preparation of modified SPINOLs by resolution, see:
- 8aM. Venugopal, S. Elango, A. Parthiban, Tetrahedron: Asymmetry 2004, 15, 3427–3431;
- 8bX. Cheng, G.-H. Hou, J.-H. Xie, Q.-L. Zhou, Org. Lett. 2004, 6, 2381–2383;
- 8cS. Chang, L. Wang, X. Lin, Org. Biomol. Chem. 2018, 16, 2239–2247;
- 8dG.-Q. Chen, B.-J. Lin, J.-M. Huang, L.-Y. Zhao, Q.-S. Chen, S.-P. Jia, Q. Yin, X. Zhang, J. Am. Chem. Soc. 2018, 140, 8064–8068.
- 9S. Li, J.-W. Zhang, X.-L. Li, D.-J. Cheng, B. Tan, J. Am. Chem. Soc. 2016, 138, 16561–16566.
- 10Z. Zheng, Y. Cao, Q. Chong, Z. Han, J. Ding, C. Luo, Z. Wang, D. Zhu, Q.-L. Zhou, K. Ding, J. Am. Chem. Soc. 2018, 140, 10374–10381.
- 11For reviews, see:
- 11aT. Jia, P. Cao, J. Liao, Chem. Sci. 2018, 9, 546–559;
- 11bM. M. Heravi, M. Dehghani, V. Zadsirjan, Tetrahedron: Asymmetry 2016, 27, 513–588;
- 11cP. Tian, H.-Q. Dong, G.-Q. Lin, ACS Catal. 2012, 2, 95–119;
- 11dG. Berthon, T. Hayashi in Catalytic Asymmetric Conjugate Reactions (Ed.: ), Wiley-VCH, Weinheim, 2010, pp. 1–70;
- 11eH. J. Edwards, J. D. Hargrave, S. D. Penrose, C. G. Frost, Chem. Rev. 2010, 110, 2093–2105;
- 11fT. Hayashi, K. Yamasaki, Chem. Rev. 2003, 103, 2829–2844;
- 11gK. Fagnou, M. Lautens, Chem. Rev. 2003, 103, 169–196.
- 12N. Tokunaga, Y. Otomaru, K. Okamoto, K. Ueyama, R. Shintani, T. Hayashi, J. Am. Chem. Soc. 2004, 126, 13584–13585.
- 13
- 13aK. Okamoto, T. Hayashi, V. H. Rawal, Chem. Commun. 2009, 4815–4817;
- 13bT. Nishimura, A. Noishiki, G. C. Tsui, T. Hayashi, J. Am. Chem. Soc. 2012, 134, 5056–5059.
- 14X. Dou, Y. Lu, T. Hayashi, Angew. Chem. Int. Ed. 2016, 55, 6739–6743; Angew. Chem. 2016, 128, 6851–6855.
- 15CCDC 1870643 and 1870644 (4 a, 4 c) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 16In the scope study, the crude diarylation products 3 were obtained after simple workup (extraction and filtration through a pad of silica gel), and were directly concentrated and used for the following spirocyclization reaction without further purification.
- 17
- 17aJ. F. Teichert, B. L. Feringa, Angew. Chem. Int. Ed. 2010, 49, 2486–2528; Angew. Chem. 2010, 122, 2538–2582;
- 17bW. Fu, W. Tang, ACS Catal. 2016, 6, 4814–4858.
- 18Y. Fu, X.-X. Guo, S.-F. Zhu, A.-G. Hu, J.-H. Xie, Q.-L. Zhou, J. Org. Chem. 2004, 69, 4648–4655.
- 19M. M. Coulter, K. G. M. Kou, B. Galligan, V. M. Dong, J. Am. Chem. Soc. 2010, 132, 16330–16333.
- 20H. Zhou, W.-H. Wang, Y. Fu, J.-H. Xie, W.-J. Shi, L.-X. Wang, Q.-L. Zhou, J. Org. Chem. 2003, 68, 1582–1584.