Aromatic Charge Resonance Interaction Probed by Infrared Spectroscopy
Kuntal Chatterjee
Institut für Optik und Atomare Physik, Technische Universität Berlin, Hardenbergstr. 36, 10623 Berlin, Germany
Search for more papers by this authorProf. Dr. Yoshiteru Matsumoto
Department of Chemistry, Faculty of Science, Shizuoka University, 836 Ohya, Suruga, Shizuoka, 422-8529 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Otto Dopfer
Institut für Optik und Atomare Physik, Technische Universität Berlin, Hardenbergstr. 36, 10623 Berlin, Germany
Search for more papers by this authorKuntal Chatterjee
Institut für Optik und Atomare Physik, Technische Universität Berlin, Hardenbergstr. 36, 10623 Berlin, Germany
Search for more papers by this authorProf. Dr. Yoshiteru Matsumoto
Department of Chemistry, Faculty of Science, Shizuoka University, 836 Ohya, Suruga, Shizuoka, 422-8529 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Otto Dopfer
Institut für Optik und Atomare Physik, Technische Universität Berlin, Hardenbergstr. 36, 10623 Berlin, Germany
Search for more papers by this authorGraphical Abstract
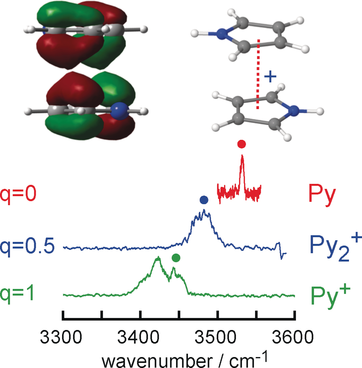
The charge distribution in π-stacked arene dimer cations is probed by infrared spectroscopy of pyrrole clusters. The linear correlation between the N−H stretch frequency and the positive partial charge on the pyrrole moiety (Py) is exploited to reveal subtle effects in the asymmetry of the charge distribution in the charge resonance interaction by symmetry reduction, induced either by substitution of functional groups or by solvation.
Abstract
Charge resonance is a strong attractive intermolecular force in aromatic dimer radical ions. Despite its importance, this fundamental interaction has not been characterized at high resolution by spectroscopy of isolated dimers. We employ vibrational infrared spectroscopy of cold aromatic pyrrole dimer cations to precisely probe the charge distribution by measuring the frequency of the isolated N−H stretch mode (νNH). We observe a linear correlation between νNH and the partial charge q on the pyrrole molecule in different environments. Subtle effects of symmetry reduction, such as substitution of functional groups (here pyrrole replaced by N-methylpyrrole) or asymmetric solvation (here by an inert N2 ligand), shift the charge distribution toward the moiety with lower ionization energy. This general approach provides a precise experimental probe of the asymmetry of the charge distribution in such aromatic homo- and heterodimer cations.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201811432-sup-0001-misc_information.pdf398.3 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. M. Salonen, M. Ellermann, F. Diederich, Angew. Chem. Int. Ed. 2011, 50, 4808–4842; Angew. Chem. 2011, 123, 4908–4944.
- 2
- 2aE. Cauët, J. Liévin, Adv. Quantum Chem. 2007, 52, 121–147;
- 2bF. Turecek, R. R. Julian, Chem. Rev. 2013, 113, 6691–6733;
- 2cA. Heckmann, C. Lambert, Angew. Chem. Int. Ed. 2012, 51, 326–392; Angew. Chem. 2012, 124, 334–404;
- 2dN. J. Hestand, F. C. Spano, Chem. Rev. 2018, 118, 7069–7163;
- 2eB. Giese, Acc. Chem. Res. 2000, 33, 631–636;
- 2fB. Giese, J. Amaudrut, A.-K. Köhler, M. Spormann, S. Wessely, Nature 2001, 412, 318;
- 2gB. Giese, Annu. Rev. Biochem. 2002, 71, 51–70;
- 2hA. A. Voityuk, M.-E. Michel-Beyerle, N. Rösch, J. Am. Chem. Soc. 1996, 118, 9750–9758.
- 3
- 3aS. Suzuki, P. G. Green, R. E. Bumgarner, S. Dasgupta, W. A. Goddard III, G. A. Blake, Science 1992, 257, 942–945;
- 3bJ. C. Ma, D. A. Dougherty, Chem. Rev. 1997, 97, 1303–1324;
- 3cD. A. Dougherty, Science 1996, 271, 163–168;
- 3dK. S. Kim, P. Tarakeshwar, J. Y. Lee, Chem. Rev. 2000, 100, 4145–4185;
- 3eR. E. Dawson, A. Hennig, D. P. Weimann, D. Emery, V. Ravikumar, J. Montenegro, T. Takeuchi, S. Gabutti, M. Mayor, J. Mareda, C. A. Schalley, S. Matile, Nat. Chem. 2010, 2, 533–538.
- 4
- 4aB. Badger, B. Brocklehurst, Nature 1968, 219, 263;
- 4bA. K. Chandra, K. Bhanuprakash, V. C. J. Bhasu, D. Srikanthan, Mol. Phys. 1984, 52, 733–741.
- 5
- 5aL. Pauling, J. Am. Chem. Soc. 1931, 53, 3225–3237;
- 5bK. D. Asmus, Acc. Chem. Res. 1979, 12, 436–442;
- 5cN. C. Baird, J. Chem. Educ. 1977, 54, 291–293;
- 5dT. Clark, J. Am. Chem. Soc. 1988, 110, 1672–1678;
- 5eP. M. W. Gill, L. Radom, J. Am. Chem. Soc. 1988, 110, 4931–4941;
- 5fP. C. Hiberty, S. Humbel, P. Archirel, J. Phys. Chem. 1994, 98, 11697–11704;
- 5gT. Clark, ChemPhysChem 2017, 18, 2766–2771.
- 6
- 6aJ. B. Birks, Nature 1967, 214, 1187–1190;
- 6bP. Ottiger, H. Köppel, S. Leutwyler, Chem. Sci. 2015, 6, 6059–6068;
- 6cJ. A. Frey, C. Holzer, W. Klopper, S. Leutwyler, Chem. Rev. 2016, 116, 5614–5641;
- 6dM. Miyazaki, M. Fujii, Phys. Chem. Chem. Phys. 2017, 19, 22759–22776.
- 7
- 7aI. C. Lewis, L. S. Singer, J. Chem. Phys. 1965, 43, 2712–2727;
- 7bO. W. Howarth, G. K. Fraenkel, J. Am. Chem. Soc. 1966, 88, 4514–4515;
- 7cH. van Willigen, E. de Boer, J. T. Cooper, W. F. Forbes, J. Chem. Phys. 1968, 49, 1190–1192;
- 7dJ. K. Kochi, R. Rathore, P. L. Maguères, J. Org. Chem. 2000, 65, 6826–6836.
- 8
- 8aB. Badger, B. Brocklehurst, R. D. Russell, Chem. Phys. Lett. 1967, 1, 122–124;
- 8bB. Badger, B. Brocklehurst, Trans. Faraday Soc. 1969, 65, 2582–2587;
- 8cB. Badger, B. Brocklehurst, Trans. Faraday Soc. 1970, 66, 2939–2947.
- 9
- 9aM. Meot-Ner, P. Hamlet, E. P. Hunter, F. H. Field, J. Am. Chem. Soc. 1978, 100, 5466–5471;
- 9bM. S. El-Shall, M. Meot-Ner, J. Phys. Chem. 1987, 91, 1088–1095;
- 9cM. Meot-Ner, J. Phys. Chem. 1980, 84, 2724–2728;
- 9dM. Rusyniak, Y. Ibrahim, E. Alsharaeh, M. Meot-Ner, M. S. El-Shall, J. Phys. Chem. A 2003, 107, 7656–7666.
- 10
- 10aK. Ohashi, N. Nishi, J. Phys. Chem. 1992, 96, 2931–2932;
- 10bK. Ohashi, Y. Nakai, T. Shibata, N. Nishi, Laser Chem. 1994, 14, 3–14;
- 10cK. Ohashi, Y. Inokuchi, N. Nishi, Chem. Phys. Lett. 1996, 263, 167–172;
- 10dY. Inokuchi, N. Nishi, J. Chem. Phys. 2001, 114, 7059–7065.
- 11Y. Inokuchi, K. Ohashi, M. Matsumoto, N. Nishi, J. Phys. Chem. 1995, 99, 3416–3418.
- 12K. Ohashi, Y. Nakane, Y. Inokuchi, Y. Nakai, N. Nishi, Chem. Phys. 1998, 239, 429–436.
- 13
- 13aY. Inokuchi, K. Ohashi, H. Sekiya, N. Nishi, J. Chem. Phys. 2002, 117, 10648–10653;
- 13bM. Matsumoto, Y. Inokuchi, K. Ohashi, N. Nishi, J. Phys. Chem. A 1997, 101, 4574–4578.
- 14
- 14aS. Chakraborty, A. Patzer, A. Lagutschenkov, J. Langer, O. Dopfer, Int. J. Mass Spectrom. 2010, 297, 85–95;
- 14bA. Fujii, E. Fujimaki, T. Ebata, N. Mikami, J. Chem. Phys. 2000, 112, 6275–6284;
- 14cK. Chatterjee, O. Dopfer, Phys. Chem. Chem. Phys. 2017, 19, 32262–32271;
- 14dP. A. Pieniazek, S. E. Bradforth, A. I. Krylov, J. Chem. Phys. 2008, 129, 074104;
- 14eY. A. Mantz, F. L. Gervasio, T. Laino, M. Parrinello, J. Phys. Chem. A 2007, 111, 105–112.
- 15
- 15aK. Ohashi, Y. Inokuchi, N. Nishi, Chem. Phys. Lett. 1996, 257, 137–142;
- 15bK. Ohashi, Y. Inokuchi, H. Itzutsu, K. Hino, N. Yamamoto, N. Nishi, H. Sekiya, Chem. Phys. Lett. 2000, 323, 43–48.
- 16V. Profant, V. Poterya, M. Farnik, P. Slavicek, U. Buck, J. Phys. Chem. A 2007, 111, 12477–12486.
- 17M. Schütz, Y. Matsumoto, A. Bouchet, M. Öztürk, O. Dopfer, Phys. Chem. Chem. Phys. 2017, 19, 3970–3986.
- 18
- 18aN. Solcà, O. Dopfer, Angew. Chem. Int. Ed. 2002, 41, 3628–3631;
10.1002/1521-3773(20021004)41:19<3628::AID-ANIE3628>3.0.CO;2-1 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 3781–3784;
- 18bO. Dopfer, D. Roth, J. P. Maier, J. Am. Chem. Soc. 2002, 124, 494–502;
- 18cN. Solcà, O. Dopfer, J. Am. Chem. Soc. 2003, 125, 1421–1430;
- 18dN. Solcà, O. Dopfer, J. Am. Chem. Soc. 2004, 126, 1716–1725;
- 18eN. Solcà, O. Dopfer, Angew. Chem. Int. Ed. 2003, 42, 1537–1540; Angew. Chem. 2003, 115, 1575–1579;
- 18fN. Solcà, O. Dopfer, J. Am. Chem. Soc. 2004, 126, 9520;
- 18gH. S. Andrei, S. A. Nizkorodov, O. Dopfer, Angew. Chem. Int. Ed. 2007, 46, 4754–4756; Angew. Chem. 2007, 119, 4838–4840;
- 18hH. S. Andrei, N. Solca, O. Dopfer, Angew. Chem. Int. Ed. 2008, 47, 395–397; Angew. Chem. 2008, 120, 401–403;
- 18iA. Patzer, S. Chakraborty, N. Solca, O. Dopfer, Angew. Chem. Int. Ed. 2010, 49, 10145–10148; Angew. Chem. 2010, 122, 10343–10346;
- 18jA. Patzer, M. Schütz, T. Möller, O. Dopfer, Angew. Chem. Int. Ed. 2012, 51, 4925–4929; Angew. Chem. 2012, 124, 5009–5013;
- 18kM. Savoca, J. Langer, O. Dopfer, Angew. Chem. Int. Ed. 2013, 52, 1568–1571; Angew. Chem. 2013, 125, 1608–1611;
- 18lM. A. R. George, N. X. Truong, M. Savoca, O. Dopfer, Angew. Chem. Int. Ed. 2018, 57, 2919–2923; Angew. Chem. 2018, 130, 2969–2973.
- 19Y. Matsumoto, K. Honma, J. Chem. Phys. 2007, 127, 184310.
- 20C. D. Cooper, A. D. Williamson, J. C. Miller, R. N. Compton, J. Chem. Phys. 1980, 73, 1527–1537.
- 21
- 21aA. Ishitani, S. Nagakura, Mol. Phys. 1967, 12, 1–12;
- 21bA. R. Dixon, D. Khuseynov, A. Sanov, J. Chem. Phys. 2015, 143, 134306;
- 21cT. Mani, D. C. Grills, J. Phys. Chem. B 2017, 121, 7327–7335;
- 21dS. Tojo, M. Fujitsuka, T. Majima, J. Org. Chem. 2012, 77, 4932–4938.
- 22O. Dopfer, Int. Rev. Phys. Chem. 2003, 22, 437–495.
- 23M. J. Frisch et al., GAUSSIAN09, Rev. D.01, Gaussian, Inc., Wallingford CT, 2009.
- 24
- 24aK. Chatterjee, O. Dopfer, Chem. Sci. 2018, 9, 2301–2318;
- 24bK. Chatterjee, O. Dopfer, Astrophys. J. 2018, 865, 114-111-115;
- 24cA. Bouchet, M. Schütz, O. Dopfer, ChemPhysChem 2016, 17, 232–243;
- 24dK. Chatterjee, O. Dopfer, J. Chem. Phys. 2018, 149, 174315;
- 24eJ. Klyne, O. Dopfer, J. Mol. Spectrosc. 2017, 337, 124–136;
- 24fJ. Klyne, M. Miyazaki, M. Fujii, O. Dopfer, Phys. Chem. Chem. Phys. 2018, 20, 3092–3108.