Simple Alkaline-Earth Metal Catalysts for Effective Alkene Hydrogenation
Dedicated to Professor Alexander C. Filippou on the occasion of his 60th birthday.
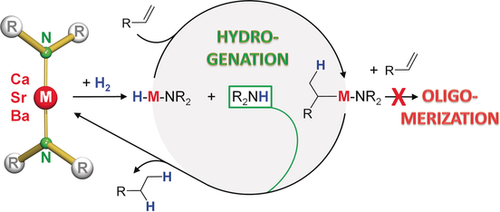
Graphical Abstract
Keep it simple: The widely used, easily accessible, alkaline earth metal amides M(NR2)2 (M=Ca, Sr, Ba, R=SiMe3) are highly active alkene hydrogenation catalysts. Alkene oligomerization is fully suppressed by trapping the reactive intermediate with R2NH. A first example of Group 2 metal hydrogen transfer catalysis is given.
Abstract
Alkaline earth metal amides (AeN′′2: Ae=Ca, Sr, Ba, N′′=N(SiMe3)2) catalyze alkene hydrogenation (80–120 °C, 1–6 bar H2, 1–10 mol % cat.), with the activity increasing with metal size. Various activated C=C bonds (styrene, p-MeO-styrene, α-Me-styrene, Ph2C=CH2, trans-stilbene, cyclohexadiene, 1-Ph-cyclohexene), semi-activated C=C bonds (Me3SiCH=CH2, norbornadiene), or non-activated (isolated) C=C bonds (norbornene, 4-vinylcyclohexene, 1-hexene) could be reduced. The results show that neutral Ca or Ba catalysts are active in the challenging hydrogenation of isolated double bonds. For activated alkenes (e.g. styrene), polymerization is fully suppressed due to fast protonation of the highly reactive benzyl intermediate by N′′H (formed in the catalyst initiation). Using cyclohexadiene as the H source, the first Ae metal catalyzed H-transfer hydrogenation is reported. DFT calculations on styrene hydrogenation using CaN′′2 show that styrene oligomerization competes with styrene hydrogenation. Calculations also show that protonation of the benzylcalcium intermediate with N′′H is a low-energy escape route, thus avoiding oligomerization.