Amide Effects in C−H Activation: Noncovalent Interactions with L-Shaped Ligand for meta Borylation of Aromatic Amides
Ranjana Bisht
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
These authors contributed equally to this work.
Search for more papers by this authorMd Emdadul Hoque
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Buddhadeb Chattopadhyay
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
Search for more papers by this authorRanjana Bisht
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
These authors contributed equally to this work.
Search for more papers by this authorMd Emdadul Hoque
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Buddhadeb Chattopadhyay
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
Search for more papers by this authorGraphical Abstract
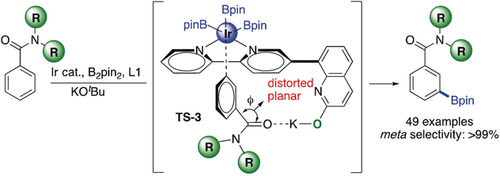
L-Shaped: A new concept for the meta borylation of aromatic amides is described. It has been shown that while esters give para borylations, amides result in meta borylations. For achieving high meta selectivity, an L-shaped ligand is used and engages in an O⋅⋅⋅K noncovalent interaction with the moderately distorted amide carbonyl group, providing exceptional control for meta C−H activation/borylation.
Abstract
A new concept for the meta-selective borylation of aromatic amides is described. It has been demonstrated that while esters gave para borylations, amides lead to meta borylations. For achieving high meta selectivity, an L-shaped bifunctional ligand has been employed and engages in an O⋅⋅⋅K noncovalent interaction with the oxygen atom of the moderately distorted amide carbonyl group. This interaction provides exceptional control for meta C−H activation/borylation.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201809929-sup-0001-misc_information.pdf8.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews on noncovalent interactions, see:
- 1aA. J. Neel, M. J. Hilton, M. S. Sigman, F. D. Toste, Nature 2017, 543, 637;
- 1bD. A. Britz, A. N. Khlobystov, Chem. Soc. Rev. 2006, 35, 637;
- 1cK. Müller-Dethlefs, P. Hobza, Chem. Rev. 2000, 100, 143.
- 2For recent leading reviews on noncovalent interactions in transition-metal catalysis, see:
- 2aC. Haldar, E. Hoque, R. Bisht, B. Chattopadhyay, Tetrahedron Lett. 2018, 59, 1269;
- 2bH. J. Davis, R. J. Phipps, Chem. Sci. 2017, 8, 864.
- 3For selected reviews, see:
- 3aM. M. Díaz-Requejo, P. J. Pérez, Chem. Rev. 2008, 108, 3379;
- 3bT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147;
- 3cJ. Li, S. De Sarkar, L. Ackerman, Top. Organomet. Chem. 2016, 55, 217;
- 3dJ. F. Hartwig, J. Am. Chem. Soc. 2016, 138, 2;
- 3eT. Gensch, M. N. Hopkinson, F. Glorius, J. Wencel-Delord, Chem. Soc. Rev. 2016, 45, 2900;
- 3fY.-F. Yang, X. Hong, J.-Q. Yu, K. N. Houk, Acc. Chem. Res. 2017, 50, 2853;
- 3gZ. Dong, Z. Ren, S. J. Thompson, Y. Xu, G. Dong, Chem. Rev. 2017, 117, 9333;
- 3hM. T. Mihai, G. R. Genov, R. J. Phipps, Chem. Soc. Rev. 2018, 47, 149;
- 3iC. Sambiagio, D. Schonbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes, M. Schnurch, Chem. Soc. Rev. 2018, 47, 6603.
- 4
- 4aD. G. Hall, Boronic Acids, Wiley-VCH, Weinheim, 2005;
10.1002/3527606548 Google Scholar
- 4bC. M. Crudden, B. W. Glasspoole, C. J. Lata, Chem. Commun. 2009, 6704.
- 5For a review, see: I. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy, J. F. Hartwig, Chem. Rev. 2010, 110, 890.
- 6For recent reviews on directed C−H borylation, see:
- 6aA. Ros, R. Fernandez, J. M. Lassaletta, Chem. Soc. Rev. 2014, 43, 3229;
- 6bL. Xu, G. Wang, S. Zhang, H. Wang, L. Wang, L. Liu, J. Jiao, P. Li, Tetrahedron 2017, 73, 7123.
- 7For borylation by directed ortho metalation, see:
- 7aM. C. Whisler, S. MacNeil, V. Snieckus, P. Beak, Angew. Chem. Int. Ed. 2004, 43, 2206; Angew. Chem. 2004, 116, 2256;
- 7bT. E. Hurst, T. K. Macklin, M. Becker, E. Hartmann, W. Kügel, J. C. Parisienne-La Salle, A. S. Batsanov, T. B. Marder, V. Snieckus, Chem. Eur. J. 2010, 16, 8155.
- 8Sterically directed meta borylations:
- 8aR. E. Maleczka, F. Shi, D. Holmes, M. R. Smith III, J. Am. Chem. Soc. 2003, 125, 7792;
- 8bJ. M. Murphy, X. Liao, J. F. Hartwig, J. Am. Chem. Soc. 2007, 129, 15434;
- 8cC. N. Iverson, M. R. Smith III, J. Am. Chem. Soc. 1999, 121, 7696;
- 8dJ. Y. Cho, C. N. Iverson, M. R. Smith III, J. Am. Chem. Soc. 2000, 122, 12868;
- 8eJ. Y. Cho, M. K. Tse, D. Holmes, R. E. Maleczka, Jr., M. R. Smith III, Science 2002, 295, 305;
- 8fG. A. Chotana, M. A. Rak, M. R. Smith III, J. Am. Chem. Soc. 2005, 127, 10539;
- 8gS. Konishi, S. Kawamorita, T. Iwai, P. G. Steel, T. B. Marder, M. Sawamura, Chem. Asian J. 2014, 9, 434.
- 9For meta borylations using noncovalent interactions, see:
- 9aY. Kuninobu, H. Ida, M. Nishi, M. Kanai, Nat. Chem. 2015, 7, 712;
- 9bR. Bisht, B. Chattopadhyay, J. Am. Chem. Soc. 2016, 138, 84;
- 9cH. J. Davis, M. T. Madalina, R. J. Phipps, J. Am. Chem. Soc. 2016, 138, 12759;
- 9dH. J. Davis, G. R. Genow, R. J. Phipps, Angew. Chem. Int. Ed. 2017, 56, 13351; Angew. Chem. 2017, 129, 13536;
- 9eM. T. Mihai, H. J. Davis, G. R. Genov, R. J. Phipps, ACS Catal. 2018, 8, 3764.
- 10Sterically directed para borylation:
- 10aY. Saito, Y. Segawa, K. Itami, J. Am. Chem. Soc. 2015, 137, 5193;
- 10bL. Yang, S. Kazuhiko, Y. Nakao, Angew. Chem. Int. Ed. 2017, 56, 4853; Angew. Chem. 2017, 129, 4931.
- 11For para borylation using noncovalent interactions, see: E. Hoque, R. Bisht, C. Haldar, B. Chattopadhyay, J. Am. Chem. Soc. 2017, 139, 7745.
- 12For applications, see: Y. Feng, D. Holte, J. Zoller, S. Umemiya, L. R. Simke, P. S. Baran, J. Am. Chem. Soc. 2015, 137, 10160.
- 13
- 13aV. R. Pattabiraman, J. W. Bode, Nature 2011, 480, 471;
- 13bE. Valeur, M. Bradley, Chem. Soc. Rev. 2009, 38, 606.
- 14V. Pace, W. Holzer, B. Olofsson, Adv. Synth. Catal. 2014, 356, 3697.
- 15A. J. Bennet, Q. P. Wang, H. Slebocka-Tilk, V. Somayaji, R. S. Brown, B. D. Santarsiero, J. Am. Chem. Soc. 1990, 112, 6383.
- 16Except for the twisted amides, in which structural constraints inhibit co-planarity and hence delocalization.
- 17
- 17aG. Meng, S. Shi, R. Lalancette, R. Szostak, M. Szostak, J. Am. Chem. Soc. 2018, 140, 727;
- 17bS. Adachi, N. Kumagai, M. Shibasaki, Tetrahedron Lett. 2018, 59, 1147.
- 18For K+ ion and carbonyl oxygen interactions, see:
- 18aA. J. Geddes, Biochem. Educ. 1980, 8, 109;
- 18bS. Y. Noskov, S. Bernèche, B. Roux, Nature 2004, 431, 830.
- 19K. B. Girma, V. Lorenz, S. Blaurock, F. T. Edelmann, Coord. Chem. Rev. 2005, 249, 1283.
- 20Ratio of the Ir catalyst, ligand, and KOtBu is very important to achieve high meta selectivity. For rate acceleration by KOtBu in iridium catalyzed borylation, see:
- 20aM. N. Eliseeva, L. T. Scott, J. Am. Chem. Soc. 2012, 134, 15169;
- 20bT. Ohmura, T. Torigoe, M. Suginome, Chem. Commun. 2014, 50, 6333;
- 20cL. Ji, K. Fucke, S. K. Bose, T. B. Marder, J. Org. Chem. 2015, 80, 661.
- 21For classic Ir/dtbpy borylations of π-electron accepting and donating substrates, see: H. Tajuddin, P. Harrisson, B. Bitterlich, J. C. Collings, N. Sim, A. S. Batsanov, M. S. Cheung, S. Kawamorita, A. C. Maxwell, L. Shukla, J. Morris, Z. Lin, T. B. Marder, P. G. Steel, Chem. Sci. 2012, 3, 3505.
- 22For a leading example, see: E. Bisz, A. Piontek, B. Dziuk, R. Szostak, M. Szostak, J. Org. Chem. 2018, 83, 3159; At this moment, we are not entirely certain about the other parameters that should be considered for lower selectivity of 2-chlorobenzamide, which would be a great point for the future matter of investigations. It should be mentioned that while use of 2.0 equivalent of B2pin2 resulted lower meta selectivity, nevertheless, we did not see multiple borylations.
- 23T. M. Boller, J. M. Murphy, M. Hapke, T. Ishiyama, N. Miyaura, J. F. Hartwig, J. Am. Chem. Soc. 2005, 127, 14263.
- 24
- 24aS. Mecozzi, A. P. West, D. A. Dougherty, J. Am. Chem. Soc. 1996, 118, 2307;
- 24bR. K. Raju, J. W. G. Bloom, Y. An, S. E. Wheeler, ChemPhysChem 2011, 12, 3116;
- 24cS. E. Wheeler, Acc. Chem. Res. 2013, 46, 1029.
- 25However, we cannot completely eliminate the pathway via TS-2, which will be the subject of future studies.