Enantioselective Synthesis of Indolines, Benzodihydrothiophenes, and Indanes by C−H Insertion of Donor/Donor Carbenes
Lucas W. Souza
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. Richard A. Squitieri
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
These authors contributed equally to this work.
Search for more papers by this authorChristine A. Dimirjian
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
Search for more papers by this authorBlanka M. Hodur
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
Search for more papers by this authorLeslie A. Nickerson
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
Search for more papers by this authorCorinne N. Penrod
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
Search for more papers by this authorProf. Jesus Cordova
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
Search for more papers by this authorDr. James C. Fettinger
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
Search for more papers by this authorCorresponding Author
Prof. Jared T. Shaw
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
Search for more papers by this authorLucas W. Souza
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. Richard A. Squitieri
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
These authors contributed equally to this work.
Search for more papers by this authorChristine A. Dimirjian
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
Search for more papers by this authorBlanka M. Hodur
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
Search for more papers by this authorLeslie A. Nickerson
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
Search for more papers by this authorCorinne N. Penrod
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
Search for more papers by this authorProf. Jesus Cordova
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
Search for more papers by this authorDr. James C. Fettinger
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
Search for more papers by this authorCorresponding Author
Prof. Jared T. Shaw
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA, 95616 USA
Search for more papers by this authorGraphical Abstract
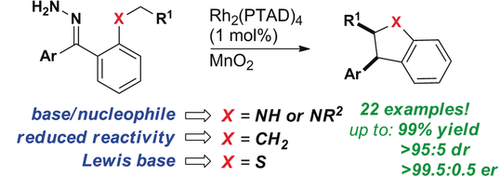
The Carbene Abides: Rhodium carbenes derived from substrates with no electron-withdrawing groups enable the highly selective syntheses of indolines and benzodihydrothiophenes. The reactions reported here represent the broadest level of functional-group tolerance yet demonstrated for enantioselective C−H insertion. A single catalyst/oxidant system is employed for all substrates and in most cases C−H insertion proceeds as a one-pot process from the hydrazone.
Abstract
We employ a single catalyst/oxidant system to enable the asymmetric syntheses of indolines, benzodihydrothiophenes, and indanes by C−H insertion of donor/donor carbenes. This methodology enables the rapid construction of densely substituted five-membered rings that form the core of many drug targets and natural products. Furthermore, oxidation of hydrazones to the corresponding diazo compounds proceeds in situ, enabling a relatively facile one- or two-pot protocol in which isolation of potentially explosive diazo alkanes is avoided. Regioselectivity studies were performed to determine the impact of sterics and electronics in donor/donor metal carbene C−H insertions to form indolines. This methodology was applied to a variety of substrates in high yield, diastereomeric, and enantiomeric ratios and to the synthesis of a patented indane estrogen receptor agonist with anti-cancer activity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201809344-sup-0001-misc_information.pdf8.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aH. M. L. Davies, R. E. J. Beckwith, Chem. Rev. 2003, 103, 2861–2904;
- 1bH. M. L. Davies, T. Hansen, J. Am. Chem. Soc. 1997, 119, 9075–9076;
- 1cH. M. L. Davies, D. Morton, Chem. Soc. Rev. 2011, 40, 1857–1869;
- 1dM. P. Doyle, R. Duffy, M. Ratnikov, L. Zhou, Chem. Rev. 2010, 110, 704–724;
- 1eM. P. Doyle, M. Ratnikov, Y. Liu, Org. Biomol. Chem. 2011, 9, 4007–4016;
- 1fJ. Egger, E. M. Carreira, Nat. Prod. Rep. 2014, 31, 449–455;
- 1gS. Zhu, D. Zhu, L. Chen, H. Zhang, Z. Ma, H. Jiang, Angew. Chem. Int. Ed. 2018, 57, 12405–12409; Angew. Chem. 2018, 130, 12585–12589.
- 2
- 2aA. Demonceau, A. F. Noels, A. J. Hubert, P. Teyssié, Chem. Commun. 1981, 688–689;
- 2bA. Demonceau, A. F. Noels, A. J. Hubert, P. Teyssié, Bull. Soc. Chim. Belg. 1984, 93, 945–948.
- 3
- 3aC. Soldi, K. N. Lamb, R. A. Squitieri, M. González-López, M. J. Di Maso, J. T. Shaw, J. Am. Chem. Soc. 2014, 136, 15142–15145;
- 3bK. N. Lamb, R. A. Squitieri, S. R. Chintala, A. J. Kwong, E. I. Balmond, C. Soldi, O. Dmitrenko, M. Castiñeira Reis, R. Chung, J. B. Addison, J. C. Fettinger, J. E. Hein, D. J. Tantillo, J. M. Fox, J. T. Shaw, Chem. Eur. J. 2017, 23, 11843–11855.
- 4
- 4aY.-B. Zhang, X.-L. Zhang, N.-H. Chen, Z.-N. Wu, W.-C. Ye, Y.-L. Li, G.-C. Wang, Org. Lett. 2017, 19, 424–427;
- 4bI. Ngantchou, B. Nyasse, C. Denier, C. Blonski, V. Hannaert, B. Schneider, Bioorg. Med. Chem. Lett. 2010, 20, 3495–3498;
- 4cL. Dejon, H. Mohammed, P. Du, C. Jacob, A. Speicher, MedChemComm 2013, 4, 1580–1583.
- 5
- 5aD. Solé, F. Mariani, M. L. Bennasar, I. Fernández, Angew. Chem. Int. Ed. 2016, 55, 6467–6470; Angew. Chem. 2016, 128, 6577–6580;
- 5bD. Zhu, J. Ma, K. Luo, H. Fu, L. Zhang, S. Zhu, Angew. Chem. Int. Ed. 2016, 55, 8452–8456; Angew. Chem. 2016, 128, 8592–8596.
- 6
- 6aR. Garner, Tetrahedron Lett. 1968, 9, 221–224;
10.1016/S0040-4039(00)75592-4 Google Scholar
- 6bN. Krogsgaard-Larsen, M. Begtrup, M. M. Herth, J. Kehler, Synthesis 2010, 4287–4299;
- 6cS. Lee, H.-J. Lim, K. L. Cha, G. A. Sulikowski, Tetrahedron 1997, 53, 16521–16532;
- 6dH.-J. Lim, G. A. Sulikowski, J. Org. Chem. 1995, 60, 2326–2327;
- 6eS. J. Mahoney, E. Fillion, Chem. Eur. J. 2012, 18, 68–71;
- 6fR. Reddy Annapureddy, C. Y. Zhou, Z. Guo, J. Wei, C. M. Che, Angew. Chem. Int. Ed. 2014, 53, 14175–14180; Angew. Chem. 2014, 126, 14399–14404;
- 6gM. Santi, S. T. R. Mueller, A. A. Folgueiras-Amador, A. Uttry, P. Hellier, T. Wirth, Eur. J. Org. Chem. 2017, 1889–1893;
- 6hG. A. Sulikowski, S. Lee, Tetrahedron Lett. 1999, 40, 8035–8038.
- 7H. M. L. Davies, T. Hansen, M. R. Churchill, J. Am. Chem. Soc. 2000, 122, 3063–3070.
- 8CCDC 1566698 and 1565066 (2 c, 9 d) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 9
- 9aC. Werlé, R. Goddard, A. Fürstner, Angew. Chem. Int. Ed. 2015, 54, 15452–15456; Angew. Chem. 2015, 127, 15672–15676;
- 9bC. Werlé, R. Goddard, P. Philipps, C. Farès, A. Fürstner, J. Am. Chem. Soc. 2016, 138, 3797–3805.
- 10E. Nakamura, N. Yoshikai, M. Yamanaka, J. Am. Chem. Soc. 2002, 124, 7181–7192.
- 11
- 11aZ. He, H. J. Shrives, J. A. Fernández-Salas, A. Abengózar, J. Neufeld, K. Yang, A. P. Pulis, D. J. Procter, Angew. Chem. Int. Ed. 2018, 57, 5759–5764; Angew. Chem. 2018, 130, 5861–5866;
- 11bW. Li, C. Schlepphorst, C. Daniliuc, F. Glorius, Angew. Chem. Int. Ed. 2016, 55, 3300–3303; Angew. Chem. 2016, 128, 3361–3364;
- 11cP. Tosatti, A. Pfaltz, Angew. Chem. Int. Ed. 2017, 56, 4579–4582; Angew. Chem. 2017, 129, 4650–4653;
- 11dS. Urban, B. Beiring, N. Ortega, D. Paul, F. Glorius, J. Am. Chem. Soc. 2012, 134, 15241–15244.
- 12
- 12aY. Harayama, M. Yoshida, D. Kamimura, Y. Wada, Y. Kita, Chem. Eur. J. 2006, 12, 4893–4899;
- 12bH. Tohma, Y. Harayama, M. Hashizume, M. Iwata, Y. Kiyono, M. Egi, Y. Kita, J. Am. Chem. Soc. 2003, 125, 11235–11240;
- 12cY. Wada, Y. Harayama, D. Kamimura, M. Yoshida, T. Shibata, K. Fujiwara, K. Morimoto, H. Fujioka, Y. Kita, Org. Biomol. Chem. 2011, 9, 4959–4976;
- 12dY. Zou, M. T. Hamann, Org. Lett. 2013, 15, 1516–1519.
- 13P. S. Skerry, N. A. Swain, D. C. Harrowven, D. Smyth, G. Bruton, R. C. D. Brown, Chem. Commun. 2004, 1772–1773.
- 14V. Aggarwal, J. Richardson, Sci. Synth. 2004, 27, 21–104.
- 15M. Vilums, J. Heuberger, L. H. Heitman, A. P. Ijzerman, Med. Res. Rev. 2015, 35, 1097–1126.
- 16
- 16aX.-D. Hao, J. Chang, B.-Y. Qin, C. Zhong, Z.-B. Chu, J. Huang, W.-J. Zhou, X. Sun, Eur. J. Med. Chem. 2015, 102, 26–38;
- 16bM. G. Schrems, E. Neumann, A. Pfaltz, Angew. Chem. Int. Ed. 2007, 46, 8274–8276; Angew. Chem. 2007, 119, 8422–8424;
- 16cZ. Zhang, J. Wang, J. Li, F. Yang, G. Liu, W. Tang, W. He, J.-J. Fu, Y.-H. Shen, A. Li, W.-D. Zhang, J. Am. Chem. Soc. 2017, 139, 5558–5567.
- 17
- 17aB. Hong, C. Li, Z. Wang, J. Chen, H. Li, X. Lei, J. Am. Chem. Soc. 2015, 137, 11946–11949;
- 17bH. Saito, H. Oishi, S. Kitagaki, S. Nakamura, M. Anada, S. Hashimoto, Org. Lett. 2002, 4, 3887–3890.
- 18“Indane estrogen receptor modulators and uses thereof”: S. P. Govek, N. D. Smith (Seragon Pharmaceuticals, Inc., USA; Aragon Pharmaceuticals, Inc.), US8853423B2, 2014.
- 19M. S. Malik, S. N. Rastogi, Indian J. Chem. Sect. B 1981, 20B, 174–175.