Non-Directed Cross-Dehydrogenative (Hetero)arylation of Allylic C(sp3)−H bonds enabled by C−H Activation
Andreas Lerchen
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorTobias Knecht
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorMaximilian Koy
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorDr. Johannes B. Ernst
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorDr. Klaus Bergander
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorDr. Constantin G. Daniliuc
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Frank Glorius
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorAndreas Lerchen
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorTobias Knecht
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorMaximilian Koy
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorDr. Johannes B. Ernst
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorDr. Klaus Bergander
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorDr. Constantin G. Daniliuc
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Frank Glorius
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorDedicated to Professor Helmut Schwarz on the occasion of his 75th birthday
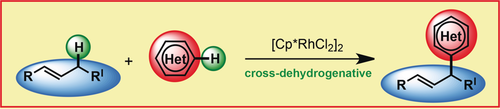
Graphical Abstract
Catch desired couplings: The non-directed cross-dehydrogenative coupling of allylic C(sp3)−H bonds with (hetero)arene C(sp2)−H bonds is enabled by C−H activation and employs abundant chemical feedstocks: olefins and (hetero)arenes. A particular highlight of this mild reaction is the applicability in late-stage functionalization reactions.
Abstract
Herein, we report the selective, non-directed and cross-dehydrogenative coupling of allylic C(sp3)−H bonds with C(sp2)−H bonds of (hetero)arenes. The methodology employs olefins and (hetero)arenes which are abundantly available chemical feedstocks, and could be applied in late-stage functionalization reactions of pharmaceuticals. Furthermore, the system exclusively delivers the allylic C−C coupling products highlighting the preservation of the olefin substitution pattern for further derivatization.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201807047-sup-0001-misc_information.pdf13.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. C. K. Chu, T. Rovis, Angew. Chem. Int. Ed. 2018, 57, 62; Angew. Chem. 2018, 130, 64;
- 1bJ. He, M. Wasa, K. S. L. Chan, Q. Shao, J.-Q. Yu, Chem. Rev. 2017, 117, 8754;
- 1cR. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer, O. Baudoin, Chem. Eur. J. 2010, 16, 2654.
- 2B. M. Trost, Science 1991, 254, 1471.
- 3
- 3aP. Gandeepan, L. Ackermann, Chem 2018, 4, 199;
- 3bY. Liu, H. Ge, Nat. Chem. 2017, 9, 26;
- 3cF.-L. Zhang, K. Hong, T.-J. Li, H. Park, J.-Q. Yu, Science 2016, 351, 252.
- 4
- 4aT. Cernak, K. D. Dykstra, S. Tyagarajan, P. Vachal, S. W. Krska, Chem. Soc. Rev. 2016, 45, 546;
- 4bJ. F. Hartwig, M. A. Larsen, ACS Cent. Sci. 2016, 2, 281;
- 4cJ. Wencel-Delord, F. Glorius, Nat. Chem. 2013, 5, 369.
- 5
- 5aC. Liu, J. Yuan, M. Gao, S. Tang, W. Li, R. Shi, A. Lei, Chem. Rev. 2015, 115, 12138;
- 5bS. A. Girard, T. Knauber, C.-J. Li, Angew. Chem. Int. Ed. 2014, 53, 74; Angew. Chem. 2014, 126, 76;
- 5cN. Kuhl, M. N. Hopkinson, J. Wencel-Delord, F. Glorius, Angew. Chem. Int. Ed. 2012, 51, 10236; Angew. Chem. 2012, 124, 10382;
- 5dC. S. Yeung, V. M. Dong, Chem. Rev. 2011, 111, 1215;
- 5eC.-J. Li, Acc. Chem. Res. 2009, 42, 335.
- 6
- 6aJ. F. Hartwig, Acc. Chem. Res. 2017, 50, 549;
- 6bS. R. Neufeldt, M. S. Sanford, Acc. Chem. Res. 2012, 45, 936.
- 7For a Highlight, see: X. Bugaut, F. Glorius, Angew. Chem. Int. Ed. 2011, 50, 7479; Angew. Chem. 2011, 123, 7618. For selected examples, see:
- 7aY. Yang, J. Lan, J. You, Chem. Rev. 2017, 117, 8787;
- 7bD. R. Stuart, K. Fagnou, Science 2007, 316, 1172;
- 7cN. Kuhl, M. N. Hopkinson, F. Glorius, Angew. Chem. Int. Ed. 2012, 51, 8230; Angew. Chem. 2012, 124, 8354.
- 8F. Romanov-Michailidis, B. D. Ravetz, D. W. Paley, T. Rovis, J. Am. Chem. Soc. 2018, 140, 5370.
- 9For selected examples, see:
- 9aR. Ma, M. C. White, J. Am. Chem. Soc. 2018, 140, 3202;
- 9bJ. S. Burman, S. B. Blakey, Angew. Chem. Int. Ed. 2017, 56, 13666; Angew. Chem. 2017, 129, 13854;
- 9cC. C. Pattillo, I. I. Strambeanu, P. Calleja, N. A. Vermeulen, T. Mizuno, M. C. White, J. Am. Chem. Soc. 2016, 138, 1265;
- 9dP.-S. Wang, M.-L. Shen, T.-C. Wang, H.-C. Lin, L.-Z. Gong, Angew. Chem. Int. Ed. 2017, 56, 16032; Angew. Chem. 2017, 129, 16248;
- 9eA. Archambeau, T. Rovis, Angew. Chem. Int. Ed. 2015, 54, 13337; Angew. Chem. 2015, 127, 13535;
- 9fT. Cochet, V. Bellosta, D. Roche, J.-Y. Ortholand, A. Greiner, J. Cossy, Chem. Commun. 2012, 48, 10745;
- 9gM. E. Harvey, D. G. Musaev, J. Du Bois, J. Am. Chem. Soc. 2011, 133, 17207;
- 9hG. Yin, Y. Wu, G. Liu, J. Am. Chem. Soc. 2010, 132, 11978;
- 9iG. Liu, G. Yin, L. Wu, Angew. Chem. Int. Ed. 2008, 47, 4733; Angew. Chem. 2008, 120, 4811;
- 9jR. C. Larock, T. R. Hightower, L. A. Hasvold, K. P. Peterson, J. Org. Chem. 1996, 61, 3584;
- 9kB. Åkermark, G. Åkermark, L. S. Hegedus, K. Zetterberg, J. Am. Chem. Soc. 1981, 103, 3037.
- 10For selected examples, see:
- 10aC. Li, M. Li, J. Li, J. Liao, W. Wu, H. Jiang, J. Org. Chem. 2017, 82, 10912;
- 10bS. E. Ammann, W. Liu, M. C. White, Angew. Chem. Int. Ed. 2016, 55, 9571; Angew. Chem. 2016, 128, 9723;
- 10cX. Xing, N. R. O'Connor, B. M. Stoltz, Angew. Chem. Int. Ed. 2015, 54, 11186; Angew. Chem. 2015, 127, 11338;
- 10dP.-S. Wang, P. Liu, Y.-J. Zhai, H.-C. Lin, Z.-Y. Han, L.-Z. Gong, J. Am. Chem. Soc. 2015, 137, 12732;
- 10eC. T. Check, W. H. Henderson, B. C. Wray, M. J. Vanden Eynden, J. P. Stambuli, J. Am. Chem. Soc. 2011, 133, 18503;
- 10fA. N. Campbell, P. B. White, I. A. Guzei, S. S. Stahl, J. Am. Chem. Soc. 2010, 132, 15116;
- 10gE. M. Stang, M. Christina White, Nat. Chem. 2009, 1, 547;
- 10hM. S. Chen, N. Prabagaran, N. A. Labenz, M. C. White, J. Am. Chem. Soc. 2005, 127, 6970;
- 10iA. Heumann, B. Åkermark, Angew. Chem. Int. Ed. Engl. 1984, 23, 453; Angew. Chem. 1984, 96, 443.
- 11For selected examples, see:
- 11aR.-B. Hu, C.-H. Wang, W. Ren, Z. Liu, S.-D. Yang, ACS Catal. 2017, 7, 7400;
- 11bH.-C. Lin, P.-S. Wang, Z.-L. Tao, Y.-G. Chen, Z.-Y. Han, L.-Z. Gong, J. Am. Chem. Soc. 2016, 138, 14354;
- 11cB. M. Trost, E. J. Donckele, D. A. Thaisrivongs, M. Osipov, J. T. Masters, J. Am. Chem. Soc. 2015, 137, 2776;
- 11dJ. M. Howell, W. Liu, A. J. Young, M. C. White, J. Am. Chem. Soc. 2014, 136, 5750;
- 11eB. M. Trost, D. A. Thaisrivongs, E. J. Donckele, Angew. Chem. Int. Ed. 2013, 52, 1523; Angew. Chem. 2013, 125, 1563;
- 11fB. M. Trost, M. M. Hansmann, D. A. Thaisrivongs, Angew. Chem. Int. Ed. 2012, 51, 4950; Angew. Chem. 2012, 124, 5034;
- 11gA. J. Young, M. C. White, J. Am. Chem. Soc. 2008, 130, 14090;
- 11hS. Lin, C.-X. Song, G.-X. Cai, W.-H. Wang, Z.-J. Shi, J. Am. Chem. Soc. 2008, 130, 12901;
- 11iL. S. Hegedus, T. Hayashi, W. H. Darlington, J. Am. Chem. Soc. 1978, 100, 7747.
- 12
- 12aJ. D. Cuthbertson, D. W. C. MacMillan, Nature 2015, 519, 74;
- 12bM. Sekine, L. Ilies, E. Nakamura, Org. Lett. 2013, 15, 714;
- 12cG.-W. Wang, A.-X. Zhou, S.-X. Li, S.-D. Yang, Org. Lett. 2014, 16, 3118;
- 12dH. Jiang, W. Yang, H. Chen, J. Li, W. Wu, Chem. Commun. 2014, 50, 7202.
- 13
- 13aJ. Le Bras, J. Muzart, Chem. Rev. 2011, 111, 1170;
- 13bL. Zhou, W. Lu, Chem. Eur. J. 2014, 20, 634.
- 14
- 14aJ. Escudero, V. Bellosta, J. Cossy, Angew. Chem. Int. Ed. 2018, 57, 574; Angew. Chem. 2018, 130, 583;
- 14bY. Shibata, E. Kudo, H. Sugiyama, H. Uekusa, K. Tanaka, Organometallics 2016, 35, 1547;
- 14cS. Rakshit, F. W. Patureau, F. Glorius, J. Am. Chem. Soc. 2010, 132, 9585. For further examples on allylic C−H amination, see: Ref. [9b,e,f]. For an example on biaryl formation, see: Ref. [7c].
- 15
- 15aJ. T. Edwards, R. R. Merchant, K. S. McClymont, K. W. Knouse, T. Qin, L. R. Malins, B. Vokits, S. A. Shaw, D.-H. Bao, F.-L. Wei, T. Zhou, M. D. Eastgate, P. S. Baran, Nature 2017, 545, 213;
- 15bA. Noble, S. J. McCarver, D. W. C. MacMillan, J. Am. Chem. Soc. 2015, 137, 624;
- 15cM. Koy, F. Sandfort, A. Tlahuext-Aca, L. Quach, C. G. Daniliuc, F. Glorius, Chem. Eur. J. 2018, 24, 4552.
- 16B. Wucher, M. Moser, S. A. Schumacher, F. Rominger, D. Kunz, Angew. Chem. Int. Ed. 2009, 48, 4417; Angew. Chem. 2009, 121, 4481.
- 17
- 17aP. A. Evans, J. D. Nelson, J. Am. Chem. Soc. 1998, 120, 5581;
- 17bB. Plietker, Angew. Chem. Int. Ed. 2006, 45, 1469; Angew. Chem. 2006, 118, 1497.
- 18CCDC 1839138 and 1839139 (3 ac and 3 fa) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.