Trapping the Complex Molecular Machinery of Polyketide and Fatty Acid Synthases with Tunable Silylcyanohydrin Crosslinkers
Dedicated to Gilbert Stork
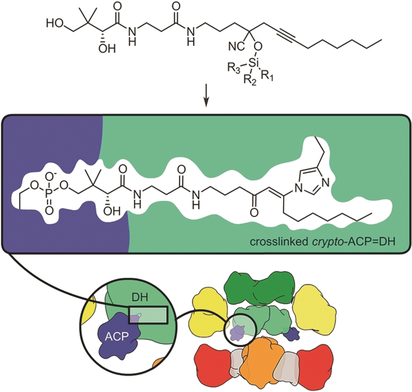
Graphical Abstract
Mechanism-based crosslinking is a vital tool to trap states within multimodular enzymatic systems. A caged system is described that applies silylcyanohydrin chemistry to mask a reactive ketone warhead. Given the diversity of different silyl groups, this system can be tuned to deliver high yields of protein–protein crosslinking as defined in the context of large polyketide and fatty acid synthases.
Abstract
Many families of natural products are synthesized by large multidomain biological machines commonly referred to as megasynthases. While the advance of mechanism-based tools has opened new windows into the structural features within the protein–protein interfaces guiding carrier protein dependent enzymes, there is an immediate need for tools that can be engaged to link co-translated domains in a site-selective manner. Now, the use of silylcyanohydrins is demonstrated in a two-step, two-site selective crosslinking for the trapping of carrier–protein interactions within megasynthases. This advance provides a new tool to trap intermediate states within multimodular systems, a key step toward understanding the specificities within fatty acid (FAS) and polyketide (PKS) synthases.