Synthesis of Triply Fused Porphyrin-Nanographene Conjugates
Qiang Chen
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany
Search for more papers by this authorDr. Luigi Brambilla
Dipartimento di Chimica, Materiali ed Ingegneria Chimica “G. Natta”, Politecnico di Milano, Piazza Leonardo da Vinci, 32, 20133 Milano, Italy
Search for more papers by this authorDr. Lakshya Daukiya
KU Leuven, Department of Chemistry, Division of Molecular Imaging and Photonics, Celestijnenlaan 200F, 3001 Leuven, Belgium
Search for more papers by this authorDr. Kunal S. Mali
KU Leuven, Department of Chemistry, Division of Molecular Imaging and Photonics, Celestijnenlaan 200F, 3001 Leuven, Belgium
Search for more papers by this authorProf. Dr. Steven De Feyter
KU Leuven, Department of Chemistry, Division of Molecular Imaging and Photonics, Celestijnenlaan 200F, 3001 Leuven, Belgium
Search for more papers by this authorProf. Matteo Tommasini
Dipartimento di Chimica, Materiali ed Ingegneria Chimica “G. Natta”, Politecnico di Milano, Piazza Leonardo da Vinci, 32, 20133 Milano, Italy
Search for more papers by this authorCorresponding Author
Prof. Dr. Klaus Müllen
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany
Search for more papers by this authorCorresponding Author
Dr. Akimitsu Narita
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany
Search for more papers by this authorQiang Chen
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany
Search for more papers by this authorDr. Luigi Brambilla
Dipartimento di Chimica, Materiali ed Ingegneria Chimica “G. Natta”, Politecnico di Milano, Piazza Leonardo da Vinci, 32, 20133 Milano, Italy
Search for more papers by this authorDr. Lakshya Daukiya
KU Leuven, Department of Chemistry, Division of Molecular Imaging and Photonics, Celestijnenlaan 200F, 3001 Leuven, Belgium
Search for more papers by this authorDr. Kunal S. Mali
KU Leuven, Department of Chemistry, Division of Molecular Imaging and Photonics, Celestijnenlaan 200F, 3001 Leuven, Belgium
Search for more papers by this authorProf. Dr. Steven De Feyter
KU Leuven, Department of Chemistry, Division of Molecular Imaging and Photonics, Celestijnenlaan 200F, 3001 Leuven, Belgium
Search for more papers by this authorProf. Matteo Tommasini
Dipartimento di Chimica, Materiali ed Ingegneria Chimica “G. Natta”, Politecnico di Milano, Piazza Leonardo da Vinci, 32, 20133 Milano, Italy
Search for more papers by this authorCorresponding Author
Prof. Dr. Klaus Müllen
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany
Search for more papers by this authorCorresponding Author
Dr. Akimitsu Narita
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany
Search for more papers by this authorGraphical Abstract
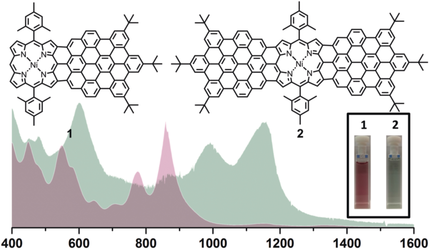
Triple whammy: Triply fused porphyrin-nanographene conjugates (1 and 2) have been synthesized by the Scholl reaction using tailor-made porphyrin based precursors. The conjugates show broad and intense near-infrared absorption. The self-assembled bilayer of 2 was observed at the trichlorobenzene/highly oriented pyrolytic graphite (HOPG) interface.
Abstract
Two unprecedented porphyrin fused nanographene molecules, 1 and 2, have been synthesized by the Scholl reaction from tailor-made precursors based on benzo[m]tetraphene-substituted porphyrins. The chemical structures were validated by a combination of high-resolution matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (HR MALDI-TOF MS), IR and Raman spectroscopy, and scanning tunnelling microscopy (STM). The UV-vis-near infrared absorption spectroscopy of 1 and 2 demonstrated broad and largely red-shifted absorption spectra extending up to 1000 and 1400 nm, respectively, marking the significant extension of the π-conjugated systems.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201805063-sup-0001-misc_information.pdf5.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Stepien, E. Gonka, M. Zyla, N. Sprutta, Chem. Rev. 2017, 117, 3479–3716;
- 1bT. D. Lash, T. M. Werner, M. L. Thompson, J. M. Manley, J. Org. Chem. 2001, 66, 3152–3159;
- 1cH. S. Gill, M. Harmjanz, J. Santamaria, I. Finger, M. J. Scott, Angew. Chem. Int. Ed. 2004, 43, 485–490; Angew. Chem. 2004, 116, 491–496;
- 1dY. Nakamura, N. Aratani, H. Shinokubo, A. Takagi, T. Kawai, T. Matsumoto, Z. S. Yoon, D. Y. Kim, T. K. Ahn, D. Kim, A. Muranaka, N. Kobayashi, A. Osuka, J. Am. Chem. Soc. 2006, 128, 4119–4127;
- 1eS. Hiroto, Y. Miyake, H. Shinokubo, Chem. Rev. 2017, 117, 2910–3043;
- 1fT. Tanaka, A. Osuka, Chem. Soc. Rev. 2015, 44, 943–969;
- 1gH. Mori, T. Tanaka, A. Osuka, J. Mater. Chem. C 2013, 1, 2500;
- 1hA. Osuka, T. Tanaka, Chem. Eur. J. 2018, https://doi.org/10.1002/chem.201802810.
- 2C. Jiao, N. Zu, K. W. Huang, P. Wang, J. Wu, Org. Lett. 2011, 13, 3652–3655.
- 3M. H. Sayyad, M. Saleem, K. S. Karimov, M. Yaseen, M. Ali, K. Y. Cheong, A. F. Mohd Noor, Appl. Phys. A 2010, 98, 103–109.
- 4N. Aratani, D. Kim, A. Osuka, Chem. Asian J. 2009, 4, 1172–1182.
- 5
- 5aL. Barloy, D. Dolphin, D. Dupre, T. P. Wijesekera, J. Org. Chem. 1994, 59, 7976–7985;
- 5bS. Richeter, C. Jeandon, J.-P. Gisselbrecht, R. Ruppert, H. J. Callot, J. Am. Chem. Soc. 2002, 124, 6168–6179;
- 5cS. Fox, R. W. Boyle, Chem. Commun. 2004, 1322–1323;
- 5dD. M. Shen, C. Liu, Q. Y. Chen, Chem. Commun. 2005, 4982–4984;
- 5eD. M. Shen, C. Liu, Q. Y. Chen, J. Org. Chem. 2006, 71, 6508–6511.
- 6
- 6aA. N. Cammidge, P. J. Scaife, G. Berber, D. L. Hughes, Org. Lett. 2005, 7, 3413–3416;
- 6bM. Tanaka, S. Hayashi, S. Eu, T. Umeyama, Y. Matano, H. Imahori, Chem. Commun. 2007, 2069–2071.
- 7
- 7aO. Yamane, K.-i. Sugiura, H. Miyasaka, K. Nakamura, T. Fujimoto, K. Nakamura, T. Kaneda, Y. Sakata, M. Yamashita, Chem. Lett. 2004, 33, 40–41;
- 7bV. V. Diev, K. Hanson, J. D. Zimmerman, S. R. Forrest, M. E. Thompson, Angew. Chem. Int. Ed. 2010, 49, 5523–5526; Angew. Chem. 2010, 122, 5655–5658.
- 8K. Kurotobi, K. S. Kim, S. B. Noh, D. Kim, A. Osuka, Angew. Chem. Int. Ed. 2006, 45, 3944–3947; Angew. Chem. 2006, 118, 4048–4051.
- 9K. Ota, T. Tanaka, A. Osuka, Org. Lett. 2014, 16, 2974–2977.
- 10V. V. Diev, C. W. Schlenker, K. Hanson, Q. Zhong, J. D. Zimmerman, S. R. Forrest, M. E. Thompson, J. Org. Chem. 2012, 77, 143–159.
- 11
- 11aN. K. Davis, M. Pawlicki, H. L. Anderson, Org. Lett. 2008, 10, 3945–3947;
- 11bN. K. Davis, A. L. Thompson, H. L. Anderson, Org. Lett. 2010, 12, 2124–2127;
- 11cN. K. Davis, A. L. Thompson, H. L. Anderson, J. Am. Chem. Soc. 2011, 133, 30–31.
- 12L. Chen, Y. Hernandez, X. Feng, K. Mullen, Angew. Chem. Int. Ed. 2012, 51, 7640–7654; Angew. Chem. 2012, 124, 7758–7773.
- 13
- 13aJ. Wu, A. Fechtenkotter, J. Gauss, M. D. Watson, M. Kastler, C. Fechtenkotter, M. Wagner, K. Mullen, J. Am. Chem. Soc. 2004, 126, 11311–11321;
- 13bL. F. Dössel, V. Kamm, I. A. Howard, F. Laquai, W. Pisula, X. Feng, C. Li, M. Takase, T. Kudernac, S. De Feyter, K. Mullen, J. Am. Chem. Soc. 2012, 134, 5876–5886;
- 13cX. Feng, W. Pisula, T. Kudernac, D. Wu, L. Zhi, S. De Feyter, K. Mullen, J. Am. Chem. Soc. 2009, 131, 4439–4448;
- 13dY. Yamamoto, T. Fukushima, Y. Suna, N. Ishii, A. Saeki, S. Seki, S. Tagawa, M. Taniguchi, T. Kawai, T. Aida, Science 2006, 314, 1761–1764.
- 14S. Fujii, T. Enoki, Acc. Chem. Res. 2013, 46, 2202–2210.
- 15J. Holzwarth, K. Y. Amsharov, D. I. Sharapa, D. Reger, K. Roshchyna, D. Lungerich, N. Jux, F. Hauke, T. Clark, A. Hirsch, Angew. Chem. Int. Ed. 2017, 56, 12184–12190; Angew. Chem. 2017, 129, 12352–12358.
- 16T. Dumslaff, B. Yang, A. Maghsoumi, G. Velpula, K. S. Mali, C. Castiglioni, S. De Feyter, M. Tommasini, A. Narita, X. Feng, K. Mullen, J. Am. Chem. Soc. 2016, 138, 4726–4729.
- 17
- 17aJ. M. Englert, J. Malig, V. A. Zamolo, A. Hirsch, N. Jux, Chem. Commun. 2013, 49, 4827–4829;
- 17bD. Lungerich, J. F. Hitzenberger, M. Marcia, F. Hampel, T. Drewello, N. Jux, Angew. Chem. Int. Ed. 2014, 53, 12231–12235; Angew. Chem. 2014, 126, 12427–12431.
- 18W. Perkins, F. R. Fischer, Chem. Eur. J. 2017, 23, 17687–17691.
- 19O. B. Locos, D. P. Arnold, Org. Biomol. Chem. 2006, 4, 902–916.
- 20L. Yu, K. Muthukumaran, I. V. Sazanovich, C. Kirmaier, E. Hindin, J. R. Diers, P. D. Boyle, D. F. Bocian, D. Holten, J. S. Lindsey, Inorg. Chem. 2003, 42, 6629–6647.
- 21K. Jyothish, Q. Wang, W. Zhang, Adv. Synth. Catal. 2012, 354, 2073–2078.
- 22N. Fukui, S.-K. Lee, K. Kato, D. Shimizu, T. Tanaka, S. Lee, H. Yorimitsu, D. Kim, A. Osuka, Chem. Sci. 2016, 7, 4059–4066.
- 23A. Harriman, J. Chem. Soc. Faraday Trans. 1 1980, 76, 1978.
- 24CCDC 1835706 (20 b) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.