Formation of Agostic Structures Driven by London Dispersion
Dr. Qing Lu
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Frank Neese
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorCorresponding Author
Dr. Giovanni Bistoni
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorDr. Qing Lu
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Frank Neese
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorCorresponding Author
Dr. Giovanni Bistoni
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorGraphical Abstract
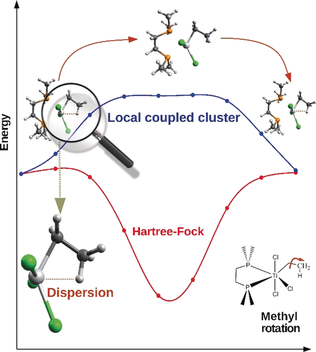
The importance of dispersion: DLPNO-CCSD(T) local energy decomposition analysis was used to elucidate the nature of β-agostic interactions. Short-range London dispersion between the agostic C−H bond and the metal center drives the formation of agostic structures to a large extent. These results were used to rationalize a series of previously published experimental findings.
Abstract
Agostic interactions between a C−H bond and a transition metal are commonly crucial in catalytic polymerization processes. Herein, a quantitative study of the nature of β-agostic interactions in a series of systems of importance in C−H bond activation reactions is reported. The analysis, characterized by the use of a coupled-cluster-based energy decomposition scheme, demonstrates that short-range London dispersion between the agostic C−H bond and the metal center plays a fundamental role in affecting the structural stability of these systems, contrary to a widely held view. These results are used to rationalize a series of previously published experimental findings.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201801531-sup-0001-misc_information.pdf1.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Brookhart, M. L. H. Green, J. Organomet. Chem. 1983, 250, 395–408;
- 1bM. Brookhart, M. L. H. Green, L. L. Wong, Prog. Inorg. Chem. 1988, 36, 1–124;
- 1cW. Scherer, G. S. McGrady, Angew. Chem. Int. Ed. 2004, 43, 1782–1806; Angew. Chem. 2004, 116, 1816–1842;
- 1dM. Brookhart, M. L. H. Green, G. Parkin, Proc. Natl. Acad. Sci. USA 2007, 104, 6908–6914.
- 2
- 2aS. Murugesan, B. Stoger, E. Pittenauer, G. Allmaier, L. F. Veiros, K. Kirchner, Angew. Chem. Int. Ed. 2016, 55, 3045–3048; Angew. Chem. 2016, 128, 3097–3100;
- 2bC. Adler, A. Bekurdts, D. Haase, W. Saak, M. Schmidtmann, R. Beckhaus, Eur. J. Inorg. Chem. 2014, 1289–1302;
- 2cA. Casitas, H. Krause, R. Goddard, A. Fürstner, Angew. Chem. Int. Ed. 2015, 54, 1521–1526; Angew. Chem. 2015, 127, 1541–1546;
- 2dL. Andrews, H. G. Cho, X. F. Wang, Angew. Chem. Int. Ed. 2005, 44, 113–116; Angew. Chem. 2005, 117, 115–118;
- 2eH. G. Cho, X. F. Wang, L. Andrews, J. Am. Chem. Soc. 2005, 127, 465–473;
- 2fD. H. Zheng, N. Wang, M. Wang, S. D. Ding, C. B. Ma, M. Y. Darensbourg, M. B. Hall, L. C. Sun, J. Am. Chem. Soc. 2014, 136, 16817–16823;
- 2gK. Ziegler, E. Holzkamp, H. Breil, H. Martin, Angew. Chem. 1955, 67, 541–547;
- 2hH. Xu, C. Hu, X. Wang, T. Diao, Organometallics 2017, 36, 4099.
- 3
- 3aP. Cossee, Tetrahedron Lett. 1960, 1, 12–16;
10.1016/S0040-4039(01)99340-2 Google Scholar
- 3bE. J. Arlman, J. Catal. 1966, 5, 178–000;
- 3cW. Kaminsky, R. Steiger, Polyhedron 1988, 7, 2375–2381;
- 3dL. H. Shultz, D. J. Tempel, M. Brookhart, J. Am. Chem. Soc. 2001, 123, 11539–11555;
- 3eH. A. De Abreu, W. B. De Almeida, H. A. Duarte, G. Fischer, T. Heine, G. Merino, G. Seifert, J. Mol. Struct. THEOCHEM 2006, 762, 9–15;
- 3fR. B. Cracknell, A. G. Orpen, J. L. Spencer, J. Chem. Soc. Chem. Commun. 1984, 326–328;
- 3gM. D. Doherty, B. Grant, P. S. White, M. Brookhart, Organometallics 2007, 26, 5950–5960.
- 4N. Miyaura, A. Suzuki, Chem. Rev. 1995, 95, 2457–2483.
- 5
- 5aM. J. S. Dewar, Bull. Soc. Chim. Fr. 1951, 18, C 71–C79;
- 5bJ. Chatt, L. A. Duncanson, J. Chem. Soc. (Resumed) 1953, 2939–2947;
- 5cG. Frenking in Modern Coordination Chemistry: The Legacy of Joseph Chatt (Eds.: ), The Royal Society of Chemistry, London, 2002, pp. 111–122.
10.1039/9781847551481-00111 Google Scholar
- 6
- 6aM. P. Mitoraj, A. Michalak, T. Ziegler, Organometallics 2009, 28, 3727–3733;
- 6bM. Lein, Coord. Chem. Rev. 2009, 253, 625–634;
- 6cW. Scherer, D. J. Wolstenholme, V. Herz, G. Eickerling, A. Bruck, P. Benndorf, P. W. Roesky, Angew. Chem. Int. Ed. 2010, 49, 2242–2246; Angew. Chem. 2010, 122, 2291–2295;
- 6dW. Scherer, V. Herz, A. Brück, C. Hauf, F. Reiner, S. Altmannshofer, D. Leusser, D. Stalke, Angew. Chem. Int. Ed. 2011, 50, 2845–2849; Angew. Chem. 2011, 123, 2897–2902;
- 6eE. Clot, O. Eisenstein, Struct. Bonding (Berlin) 2004, 113, 1–36.
- 7
- 7aG. S. McGrady, A. J. Downs, A. Haaland, W. Scherer, D. C. McKean, Chem. Commun. 1997, 1547–1548;
- 7bR. F. Jordan, P. K. Bradley, N. C. Baenziger, R. E. Lapointe, J. Am. Chem. Soc. 1990, 112, 1289–1291;
- 7cD. C. McKean, G. S. McGrady, A. J. Downs, W. Scherer, A. Haaland, Phys. Chem. Chem. Phys. 2001, 3, 2781–2794;
- 7dM. E. Thompson, S. M. Baxter, A. R. Bulls, B. J. Burger, M. C. Nolan, B. D. Santarsiero, W. P. Schaefer, J. E. Bercaw, J. Am. Chem. Soc. 1987, 109, 203–219.
- 8
- 8aE.-L. Zins, B. Silvi, M. E. Alikhani, Phys. Chem. Chem. Phys. 2015, 17, 9258–9281;
- 8bM. A. Sajjad, K. E. Christensen, N. H. Rees, P. Schwerdtfeger, J. A. Harrison, A. J. Nielson, Dalton Trans. 2017, 46, 16126–16138.
- 9
- 9aG. Frenking, N. Fröhlich, Chem. Rev. 2000, 100, 717–774;
- 9bG. Bistoni, L. Belpassi, F. Tarantelli, Angew. Chem. Int. Ed. 2013, 52, 11599–11602; Angew. Chem. 2013, 125, 11813–11816;
- 9cG. Bistoni, S. Rampino, N. Scafuri, G. Ciancaleoni, D. Zuccaccia, L. Belpassi, F. Tarantelli, Chem. Sci. 2016, 7, 1174–1184.
- 10
- 10aS. Grimme, P. R. Schreiner, Angew. Chem. Int. Ed. 2011, 50, 12639–12642; Angew. Chem. 2011, 123, 12849–12853;
- 10bJ. P. Wagner, P. R. Schreiner, Angew. Chem. Int. Ed. 2015, 54, 12274–12296; Angew. Chem. 2015, 127, 12446–12471;
- 10cS. Roesel, C. Balestrieri, P. Schreiner, Chem. Sci. 2017, 8, 405;
- 10dG. Bistoni, A. A. Auer, F. Neese, Chem. Eur. J. 2017, 23, 865–873;
- 10eD. J. Liptrot, P. P. Power, Nat. Rev. Chem. 2017, 1, 0004.
- 11S. Kristyán, P. Pulay, Chem. Phys. Lett. 1994, 229, 175–180.
- 12S. Grimme, J. Antony, S. Ehrlich, H. Krieg, J. Chem. Phys. 2010, 132, 154104.
- 13S. Grimme, Wires Comput. Mol. Sci. 2011, 1, 211–228.
- 14Z. Dawoodi, M. L. H. Green, V. S. B. Mtetwa, K. Prout, J. Chem. Soc. Chem. Commun. 1982, 802–803.
- 15
- 15aC. Riplinger, F. Neese, J. Chem. Phys. 2013, 138, 034106;
- 15bC. Riplinger, B. Sandhoefer, A. Hansen, F. Neese, J. Chem. Phys. 2013, 139, 134101;
- 15cC. Riplinger, P. Pinski, U. Becker, E. F. Valeev, F. Neese, J. Chem. Phys. 2016, 144, 024109.
- 16W. B. Schneider, G. Bistoni, M. Sparta, M. Saitow, C. Riplinger, A. A. Auer, F. Neese, J. Chem. Theory Comput. 2016, 12, 4778–4792.
- 17J. Huang, G. L. Rempel, Prog. Polym. Sci. 1995, 20, 459–526.
- 18
- 18aM. Brookhart, D. M. Lincoln, M. A. Bennett, S. Pelling, J. Am. Chem. Soc. 1990, 112, 2691–2694;
- 18bR. Xu, M. Bittner, G. Klatt, H. Koppel, J. Phys. Chem. A 2008, 112, 13139–13148;
- 18cM. Brookhart, D. M. Lincoln, A. F. Volpe, G. F. Schmidt, Organometallics 1989, 8, 1212–1218;
- 18dM. Findlater, A. Cartwright-Sykes, P. S. White, C. K. Schauer, M. Brookhart, J. Am. Chem. Soc. 2011, 133, 12274–12284;
- 18eM. Brookhart, B. E. Grant, C. P. Lenges, M. H. Prosenc, P. S. White, Angew. Chem. Int. Ed. 2000, 39, 1676–1679;
10.1002/(SICI)1521-3773(20000502)39:9<1676::AID-ANIE1676>3.0.CO;2-M CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 1742–1745.
- 19J. M. Tao, J. P. Perdew, V. N. Staroverov, G. E. Scuseria, Phys. Rev. Lett. 2003, 91, 146401.
- 20Z. Y. Lin, M. B. Hall, M. F. Guest, P. Sherwood, J. Organomet. Chem. 1994, 478, 197–203.
- 21M. Kaupp, J. Am. Chem. Soc. 1996, 118, 3018–3024.
- 22S. Rizzato, J. Berges, S. A. Mason, A. Albinati, J. Kozelka, Angew. Chem. Int. Ed. 2010, 49, 7440–7443; Angew. Chem. 2010, 122, 7602–7605.
- 23J. Thirman, M. Head-Gordon, J. Phys. Chem. Lett. 2014, 5, 1380–1385.
- 24E. Hirota, S. Saito, Y. Endo, J. Chem. Phys. 1979, 71, 1183–1187.
- 25Y. Mo, J. Gao, Acc. Chem. Res. 2007, 40, 113–119.
- 26F. Weinhold, C. R. Landis, E. D. Glendening, Int. Rev. Phys. Chem. 2016, 35, 399–440.
- 27
- 27aR. I. Papasergio, C. L. Raston, A. H. White, J. Chem. Soc. Chem. Commun. 1983, 1419–1420;
- 27bW. T. Klooster, L. Brammer, C. J. Schaverien, P. H. M. Budzelaar, J. Am. Chem. Soc. 1999, 121, 1381–1382;
- 27cW. T. Klooster, R. S. Lu, R. Anwander, W. J. Evans, T. F. Koetzle, R. Bau, Angew. Chem. Int. Ed. 1998, 37, 1268–1270;
10.1002/(SICI)1521-3773(19980518)37:9<1268::AID-ANIE1268>3.0.CO;2-Y CAS PubMed Web of Science® Google ScholarAngew. Chem. 1998, 110, 1326–1329;
- 27dW. Baratta, C. Mealli, E. Herdtweck, A. Ienco, S. A. Mason, P. Rigo, J. Am. Chem. Soc. 2004, 126, 5549–5562.
- 28F. A. Cotton, C. A. Murillo, M. A. Petrukhina, J. Organomet. Chem. 1999, 573, 78–86.