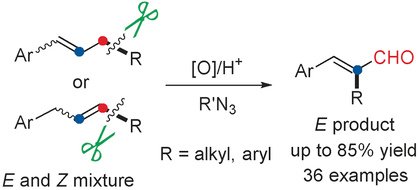
Oxygenation of Simple Olefins through Selective Allylic C−C Bond Cleavage: A Direct Approach to Cinnamyl Aldehydes
Jianzhong Liu
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
These authors contributed equally to this work.
Search for more papers by this authorXiaojin Wen
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
These authors contributed equally to this work.
Search for more papers by this authorChong Qin
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
These authors contributed equally to this work.
Search for more papers by this authorXinyao Li
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorXiao Luo
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorAo Sun
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorBencong Zhu
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorCorresponding Author
Dr. Song Song
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorCorresponding Author
Prof. Ning Jiao
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
State Key Laboratory of Organometallic Chemistry, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorJianzhong Liu
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
These authors contributed equally to this work.
Search for more papers by this authorXiaojin Wen
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
These authors contributed equally to this work.
Search for more papers by this authorChong Qin
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
These authors contributed equally to this work.
Search for more papers by this authorXinyao Li
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorXiao Luo
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorAo Sun
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorBencong Zhu
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorCorresponding Author
Dr. Song Song
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorCorresponding Author
Prof. Ning Jiao
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
State Key Laboratory of Organometallic Chemistry, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorGraphical Abstract
Cut and paste: A novel metal-free allylic C−C bond cleavage of simple unfunctionalized olefins was developed for the synthesis of valuable cinnamyl aldehyde derivatives with high regio- and stereoselectivity. This method not only provides a new approach to cinnamyl aldehydes, but also expands the application of alkyl azides as well as the recombination of olefins in organic synthesis.
Abstract
A novel metal-free allylic C−C σ-bond cleavage of simple olefins to give valuable cinnamyl aldehydes is reported. 1,2-Aryl or alkyl migration through allylic C−C bond cleavage occurs in this transformation, which is assisted by an alkyl azide reagent. This method enables O-atom incorporation into simple unfunctionalized olefins to construct cinnamyl aldehydes. The reaction features simple hydrocarbon substrates, metal-free conditions, and high regio- and stereoselectivity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201705671-sup-0001-misc_information.pdf4.7 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Reviews of C−H bond activation:
- 1aJ. He, M. Wasa, K. S. L. Chan, Q. Shao, J.-Q. Yu, Chem. Rev. 2017, 117, 8754;
- 1bY. Park, Y. Kim, S. Chang, Chem. Rev. 2017, 117, 9247;
- 1cS. R. Neufeldt, M. S. Sanford, Acc. Chem. Res. 2012, 45, 936;
- 1dK. M. Engle, T.-S. Mei, M. Wasa, J.-Q. Yu, Acc. Chem. Res. 2012, 45, 788;
- 1eA. J. Hickman, M. S. Sanford, Nature 2012, 484, 177;
- 1fH. M. L. Davies, J. R. Manning, Nature 2008, 451, 417;
- 1gR. G. Bergman, Nature 2007, 446, 391;
- 1hK. Godula, D. Sames, Science 2006, 312, 67.
- 2Reviews of C−C cleavage:
- 2aM. Murakami, N. Ishida, J. Am. Chem. Soc. 2016, 138, 13759;
- 2bL. Souillart, N. Cramer, Chem. Rev. 2015, 115, 9410;
- 2cC.-H. Jun, J.-W. Park, Top. Curr. Chem. 2014, 346, 59;
- 2dF. Chen, T. Wang, N. Jiao, Chem. Rev. 2014, 114, 8613;
- 2eA. Dermenci, J. W. Coe, G. Dong, Org. Chem. Front. 2014, 1, 567;
- 2fM. Tobisu, N. Chatani, Chem. Soc. Rev. 2008, 37, 300;
- 2gC.-H. Jun, Chem. Soc. Rev. 2004, 33, 610.
- 3Selected examples for challenging C−C bonds cleavage:
- 3aY. Xia, G. Lu, P. Liu, G. Dong, Nature 2016, 539, 546;
- 3bL. Deng, T. Xu, H. Li, G. Dong, J. Am. Chem. Soc. 2016, 138, 369;
- 3cM. H. Shaw, R. A. Croft, W. G. Whittingham, J. F. Bower, J. Am. Chem. Soc. 2015, 137, 8054;
- 3dX. Zhou, G. Dong, J. Am. Chem. Soc. 2015, 137, 13715;
- 3eL. Souillart, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 9640; Angew. Chem. 2014, 126, 9794;
- 3fH. M. Ko, G. Dong, Nat. Chem. 2014, 6, 739;
- 3gM. H. Shaw, E. Y. Melikhova, D. P. Kloer, W. G. Whittingham, J. F. Bower, J. Am. Chem. Soc. 2013, 135, 4992;
- 3hL. Liu, N. Ishida, M. Murakami, Angew. Chem. Int. Ed. 2012, 51, 2485; Angew. Chem. 2012, 124, 2535;
- 3iC. Mukai, Y. Ohta, Y. Kawaguchi, F. Inagaki, Y. Oura, J. Am. Chem. Soc. 2012, 134, 19580;
- 3jT. Seiser, O. A. Roth, N. Cramer, Angew. Chem. Int. Ed. 2009, 48, 6320; Angew. Chem. 2009, 121, 6438;
- 3kT. Seiser, N. Cramer, Angew. Chem. Int. Ed. 2008, 47, 9294; Angew. Chem. 2008, 120, 9435;
- 3lM. Murakami, T. Itahashi, Y. Ito, J. Am. Chem. Soc. 2002, 124, 13976;
- 3mC.-H. Jun, H. Lee, S.-G. Lim, J. Am. Chem. Soc. 2001, 123, 751;
- 3nC.-H. Jun, H. Lee, J. Am. Chem. Soc. 1999, 121, 880;
- 3oM. Gozin, M. Aizenberg, S.-Y. Liou, A. Weisman, Y. Ben-David, D. Milstein, Nature 1994, 370, 42;
- 3pM. Gozin, A. Weisman, Y. Ben-David, D. Milstein, Nature 1993, 364, 699;
- 3qJ. W. Suggs, C.-H. Jun, J. Am. Chem. Soc. 1986, 108, 4679.
- 4
- 4aH. M. L. Davies, J. Nikolai, Org. Biomol. Chem. 2005, 3, 4176;
- 4bT. Jensen, P. Fristrup, Chem. Eur. J. 2009, 15, 9632;
- 4cG. Liu, Y. Wu, Top. Curr. Chem. 2010, 292, 195;
- 4dA. Nakamura, M. Nakada, Synthesis 2013, 1421;
- 4eF. Liron, J. Oble, M. M. Lorion, G. Poli, Eur. J. Org. Chem. 2014, 5863.
- 5For reviews on allylic C−H activation, see:
- 5aT. A. Ramirez, B.-G. Zhao, Y. Shi, Chem. Soc. Rev. 2012, 41, 931;
- 5bB. M. Trost, M. L. Crawley, Chem. Rev. 2003, 103, 2921.
- 6
- 6aY. Wang, Z.-X. Yu, Acc. Chem. Res. 2015, 48, 2288;
- 6bP. A. Evans, D. E. Negru, D. Shang, Angew. Chem. Int. Ed. 2015, 54, 4768; Angew. Chem. 2015, 127, 4850;
- 6cP. A. Inglesby, J. Bacsa, D. E. Negru, P. A. Evans, Angew. Chem. Int. Ed. 2014, 53, 3952; Angew. Chem. 2014, 126, 4033;
- 6dX. Hong, B. M. Trost, K. N. Houk, J. Am. Chem. Soc. 2013, 135, 6588;
- 6eA. P. Dieskau, M. S. Holzwarth, B. Plietker, J. Am. Chem. Soc. 2012, 134, 5048;
- 6fM. Lin, G.-Y. Kang, Y.-A. Guo, Z.-X. Yu, J. Am. Chem. Soc. 2012, 134, 398;
- 6gS. Mazumder, D. Shang, D. E. Negru, M.-H. Baik, P. A. Evans, J. Am. Chem. Soc. 2012, 134, 20569;
- 6hA. F. G. Goldberg, B. M. Stoltz, Org. Lett. 2011, 13, 4474;
- 6iB. M. Trost, P. J. Morris, Angew. Chem. Int. Ed. 2011, 50, 6167; Angew. Chem. 2011, 123, 6291;
- 6jM. Lin, F. Li, L. Jiao, Z.-X. Yu, J. Am. Chem. Soc. 2011, 133, 1690;
- 6kH. Zhang, D. P. Curran, J. Am. Chem. Soc. 2011, 133, 10376;
- 6lP. A. Wender, B. L. Miller, Nature 2009, 460, 197;
- 6mL. Jiao, C. Yuan, Z.-X. Yu, J. Am. Chem. Soc. 2008, 130, 4421;
- 6nB. M. Trost, Y. Hu, D. B. Horne, J. Am. Chem. Soc. 2007, 129, 11781;
- 6oS. Sebelius, V. J. Olsson, K. J. Szabo, J. Am. Chem. Soc. 2005, 127, 10478;
- 6pZ.-X. Yu, P. A. Wender, K. N. Houk, J. Am. Chem. Soc. 2004, 126, 9154;
- 6qF. López, A. Delgado, J. R. Rodríguez, L. Castedo, J. L. Mascareñas, J. Am. Chem. Soc. 2004, 126, 10262;
- 6rB. M. Trost, F. D. Toste, H. Shen, J. Am. Chem. Soc. 2000, 122, 2379;
- 6sP. A. Wender, H. Takahashi, B. Witulski, J. Am. Chem. Soc. 1995, 117, 4720.
- 7
- 7aJ. Yu, H. Yan, C. Zhu, Angew. Chem. Int. Ed. 2016, 55, 1143; Angew. Chem. 2016, 128, 1155;
- 7bN. Ishida, S. Sawano, M. Murakami, Nat. Commun. 2014, 5, 3111;
- 7cN. Ishida, S. Sawano, Y. Masuda, M. Murakami, J. Am. Chem. Soc. 2012, 134, 17502;
- 7dM. Sai, H. Yorimitsu, K. Oshima, Angew. Chem. Int. Ed. 2011, 50, 3294; Angew. Chem. 2011, 123, 3352;
- 7eM. Waibel, N. Cramer, Angew. Chem. Int. Ed. 2010, 49, 4455; Angew. Chem. 2010, 122, 4557;
- 7fS. Chiba, Y.-J. Xu, Y.-F. Wang, J. Am. Chem. Soc. 2009, 131, 12886;
- 7gM. Iwasaki, S. Hayashi, K. Hirano, H. Yorimitsu, K. Oshima, J. Am. Chem. Soc. 2007, 129, 4463;
- 7hS. Hayashi, K. Hirano, H. Yorimitsu, K. Oshima, J. Am. Chem. Soc. 2006, 128, 2210;
- 7iT. Kondo, K. Kodoi, E. Nishinaga, T. Okada, Y. Morisaki, Y. Watanabe, T. Mitsudo, J. Am. Chem. Soc. 1998, 120, 5587.
- 8
- 8aSee Ref. [3i];
- 8bD. Nečas, M. Kotora, Org. Lett. 2008, 10, 5261;
- 8cD. Nečas, M. Turský, M. Kotora, J. Am. Chem. Soc. 2004, 126, 10222.
- 9Reviews of azide chemistry:
- 9aK. Shin, H. Kim, S. Chang, Acc. Chem. Res. 2015, 48, 1040;
- 9bT. Wang, N. Jiao, Acc. Chem. Res. 2014, 47, 1137;
- 9cE. Leemans, M. D'hooghe, N. De Kimpe, Chem. Rev. 2011, 111, 3268;
- 9dS. Lang, J. A. Murphy, Chem. Soc. Rev. 2006, 35, 146;
- 9eS. Bräse, C. Gil, K. Knepper, V. Zimmermann, Angew. Chem. Int. Ed. 2005, 44, 5188; Angew. Chem. 2005, 117, 5320;
- 9fV. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem. Int. Ed. 2002, 41, 2596;
10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 2708;
- 9gE. F. V. Scriven, K. Turnbull, Chem. Rev. 1988, 88, 297.
- 10Selective azidation reactions:
- 10aZ. Wu, R. Ren, C. Zhu, Angew. Chem. Int. Ed. 2016, 55, 10821; Angew. Chem. 2016, 128, 10979;
- 10bX. Huang, T. M. Bergsten, J. T. Groves, J. Am. Chem. Soc. 2015, 137, 5300;
- 10cG. Fumagalli, P. T. G. Rabet, S. Boyd, M. F. Greaney, Angew. Chem. Int. Ed. 2015, 54, 11481; Angew. Chem. 2015, 127, 11643;
- 10dR. Ren, H. Zhao, L. Huan, C. Zhu, Angew. Chem. Int. Ed. 2015, 54, 12692; Angew. Chem. 2015, 127, 12883;
- 10eA. Sharma, J. F. Hartwig, Nature 2015, 517, 600;
- 10fX. Sun, X. Li, S. Song, Y. Zhu, Y.-F. Liang, N. Jiao, J. Am. Chem. Soc. 2015, 137, 6059;
- 10gY. Wang, G.-X. Li, G. Yang, G. He, G. Chen, Chem. Sci. 2016, 7, 2679.
- 11Recent examples:
- 11aC. Jones, Q. Nguyen, T. G. Driver, Angew. Chem. Int. Ed. 2014, 53, 785; Angew. Chem. 2014, 126, 804;
- 11bT. Kang, Y. Kim, D. Lee, Z. Wang, S. Chang, J. Am. Chem. Soc. 2014, 136, 4141;
- 11cH. J. Kim, M. J. Ajitha, Y. Lee, J. Ryu, J. Kim, Y. Lee, Y. Jung, S. Chang, J. Am. Chem. Soc. 2014, 136, 1132;
- 11dH. Lu, C. Li, H. Jiang, C. L. Lizardi, X. P. Zhang, Angew. Chem. Int. Ed. 2014, 53, 7028; Angew. Chem. 2014, 126, 7148;
- 11eY.-F. Wang, G. H. Lonca, M. Le Runigo, S. Chiba, Org. Lett. 2014, 16, 4272;
- 11fJ. Wei, W. Xiao, C.-Y. Zhou, C.-M. Che, Chem. Commun. 2014, 50, 3373;
- 11gY. Shinomoto, A. Yoshimura, H. Shimizu, M. Yamazaki, V. V. Zhdankin, A. Saito, Org. Lett. 2015, 17, 5212;
- 11hM. V. Vita, J. Waser, Angew. Chem. Int. Ed. 2015, 54, 5290; Angew. Chem. 2015, 127, 5380;
- 11iE. T. Hennessy, T. A. Betley, Science 2013, 340, 591;
- 11jB. Bagh, D. L. J. Broere, V. Sinha, P. F. Kuijpers, N. P. van Leest, B. de Bruin, S. Demeshko, M. A. Siegler, J. I. van der Vlugt, J. Am. Chem. Soc. 2017, 139, 5117.
- 12
- 12aT. Liu, T.-S. Mei, J.-Q. Yu, J. Am. Chem. Soc. 2015, 137, 5871;
- 12bT. Liu, M. C. Myers, J.-Q. Yu, Angew. Chem. Int. Ed. 2017, 56, 306; Angew. Chem. 2017, 129, 312.
- 13
- 13aF. Chen, C. Qin, Y. Cui, N. Jiao, Angew. Chem. Int. Ed. 2011, 50, 11487; Angew. Chem. 2011, 123, 11689;
- 13bC. Qin, W. Zhou, F. Chen, Y. Ou, N. Jiao, Angew. Chem. Int. Ed. 2011, 50, 12595; Angew. Chem. 2011, 123, 12803.
- 14Transition-metal-catalyzed synthesis of α-substituted cinnamyl aldehydes synthesis:
- 14aP. Wang, H. Rao, F. Zhou, R. Hua, C.-J. Li, J. Am. Chem. Soc. 2012, 134, 16468;
- 14bZ. Zhang, Q. Wang, C. Chen, Z. Han, X.-Q. Dong, X.-M. Zhang, Org. Lett. 2016, 18, 3290;
- 14cM. G. Mura, L. D. Luca, M. Taddei, J. M. J. Williams, A. Porcheddu, Org. Lett. 2014, 16, 2586;
- 14dG. A. Molander, T. Fumagalli, J. Org. Chem. 2006, 71, 5743;
- 14eP. Gandeepan, P. Rajamalli, C.-H. Cheng, ACS Catal. 2014, 4, 4485;
- 14fV. Agabekov, W. Seiche, B. Breit, Chem. Sci. 2013, 4, 2418;
- 14gX. Fang, M. Zhang, R. Jackstell, M. Beller, Angew. Chem. Int. Ed. 2013, 52, 4645; Angew. Chem. 2013, 125, 4743.
- 151,3-Sigmatropic rearrangement of the allyl azido intermediate may occur:
- 15aP. A. Grieco, J. D. Clark, C. T. Jagoe, J. Am. Chem. Soc. 1991, 113, 5488;
- 15bM. Egi, Y. Yamaguchi, N. Fujiwara, S. Akai, Org. Lett. 2008, 10, 1867;
- 15cI. Nakamura, M. Owada, T. Jo, M. Terada, Org. Lett. 2017, 19, 2194.
- 16
- 16aY. Zhang, C.-J. Li, J. Am. Chem. Soc. 2006, 128, 4242;
- 16bC. Qin, N. Jiao, J. Am. Chem. Soc. 2010, 132, 15893;
- 16cY.-Z. Li, B.-J. Li, X.-Y. Lu, S. Lin, Z.-J. Shi, Angew. Chem. Int. Ed. 2009, 48, 3817; Angew. Chem. 2009, 121, 3875;
- 16dY. Zhang, C.-J. Li, Angew. Chem. Int. Ed. 2006, 45, 1949; Angew. Chem. 2006, 118, 1983.