Synthesis of Poly(arylene vinylene)s with Different End Groups by Combining Acyclic Diene Metathesis Polymerization with Wittig-type Couplings
Tomonari Miyashita
Department of Chemistry, Graduate School of Science and Engineering, Tokyo Metropolitan University, 1-1 Minami Osawa, Hachioji, Tokyo, 192-0376 Japan
These authors contributed equally to this work.
Search for more papers by this authorMikiko Kunisawa
Department of Chemistry, Graduate School of Science and Engineering, Tokyo Metropolitan University, 1-1 Minami Osawa, Hachioji, Tokyo, 192-0376 Japan
These authors contributed equally to this work.
Search for more papers by this authorDr. Shunsuke Sueki
Department of Chemistry, Graduate School of Science and Engineering, Tokyo Metropolitan University, 1-1 Minami Osawa, Hachioji, Tokyo, 192-0376 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Kotohiro Nomura
Department of Chemistry, Graduate School of Science and Engineering, Tokyo Metropolitan University, 1-1 Minami Osawa, Hachioji, Tokyo, 192-0376 Japan
Search for more papers by this authorTomonari Miyashita
Department of Chemistry, Graduate School of Science and Engineering, Tokyo Metropolitan University, 1-1 Minami Osawa, Hachioji, Tokyo, 192-0376 Japan
These authors contributed equally to this work.
Search for more papers by this authorMikiko Kunisawa
Department of Chemistry, Graduate School of Science and Engineering, Tokyo Metropolitan University, 1-1 Minami Osawa, Hachioji, Tokyo, 192-0376 Japan
These authors contributed equally to this work.
Search for more papers by this authorDr. Shunsuke Sueki
Department of Chemistry, Graduate School of Science and Engineering, Tokyo Metropolitan University, 1-1 Minami Osawa, Hachioji, Tokyo, 192-0376 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Kotohiro Nomura
Department of Chemistry, Graduate School of Science and Engineering, Tokyo Metropolitan University, 1-1 Minami Osawa, Hachioji, Tokyo, 192-0376 Japan
Search for more papers by this authorGraphical Abstract
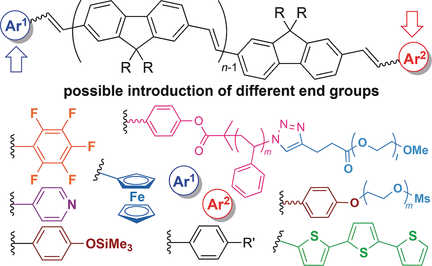
Which is the end? Conjugated polymers based on poly(9,9′-di-n-octylfluorene vinylene) with different end groups were synthesized by combining molybdenum-catalyzed acyclic diene metathesis polymerization with a subsequent Wittig-type coupling. Their fluorescence spectra were influenced by the end groups and the conjugation length.
Abstract
A series of end-functionalized poly(9,9′-di-n-octylfluorene vinylene)s (EF-PFVs) with different end groups were obtained by 1) synthesizing EF-PFV with vinyl end groups by acyclic diene metathesis (ADMET) polymerization with a molybdenum catalyst and termination with an aldehyde and 2) subsequent olefin metathesis of the vinyl group with the molybdenum catalyst followed by Wittig-type coupling with another aldehyde. The exclusive formation of EF-PFVs containing a vinyl end group by the ADMET polymerization was confirmed by grafting PEG, and by the synthesis of amphiphilic triblock copolymers by combining atom transfer radical polymerization from the PFV chain end with PEG grafting through a click reaction. Various EF-PFVs with different end groups, such as C6F5, pyridyl, ferrocenyl, and terthiophene, have thus been prepared. Their fluorescence spectra (e.g., intensities, emission wavelengths) were influenced by the end groups and the length of the conjugation.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201700466-sup-0001-misc_information.pdf2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Organic Light Emitting Devices (Eds.: ), Wiley-VCH, Weinheim, 2006;
- 1b Handbook of Conducting Polymers, 3rd ed. ), CRC, Boca Raton, 2007.
- 2For selected reviews, see:
- 2aThematic Issue on “Organic Electronic Materials and Devices”: S. R. Forrest, M. E. Thompson, Chem. Rev. 2007, 107, 923;
- 2bB. C. Thompson, J. M. J. Fréchet, Angew. Chem. Int. Ed. 2008, 47, 58; Angew. Chem. 2008, 120, 62;
- 2cS. Allard, M. Forster, B. Souharce, H. Thiem, U. Scherf, Angew. Chem. Int. Ed. 2008, 47, 4070; Angew. Chem. 2008, 120, 4138;
- 2dA. C. Grimsdale, K. L. Chan, R. E. Martin, P. G. Jokisz, A. B. Holmes, Chem. Rev. 2009, 109, 897;
- 2eThematic Issue on “Materials for Electronics”: R. D. Miller, E. A. Chandross, Chem. Rev. 2010, 110, 1;
- 2fC. Li, M. Liu, N. G. Pschirer, M. Baumgarten, K. Müllen, Chem. Rev. 2010, 110, 6817;
- 2gS. S. Zade, N. Zamoshchik, M. Bendikov, Acc. Chem. Res. 2011, 44, 14–24;
- 2hH. Zhou, L. Yang, W. You, Macromolecules 2012, 45, 607.
- 3
- 3aJ. K. Park, J. Jo, J. H. Seo, J. S. Moon, Y. D. Park, K. Lee, A. J. Heeger, G. C. Bazan, Adv. Mater. 2011, 23, 2430;
- 3bQ. Wang, B. Zhang, L. Liu, Y. Chen, Y. Qu, X. Zhang, J. Yang, Z. Xie, Y. Geng, L. Wang, F. Wang, J. Phys. Chem. C 2012, 116, 21727.
- 4
- 4aT. Miteva, A. Meisel, W. Knoll, H. G. Nothofer, U. Scherf, D. C. Müller, K. Meerholz, A. Yasuda, D. Neher, Adv. Mater. 2001, 13, 565;
- 4bC. Ego, D. Marsitzky, S. Becker, J. Zhang, A. C. Grimsdale, K. Müllen, J. D. MacKenzie, C. Silva, R. H. Friend, J. Am. Chem. Soc. 2003, 125, 437;
- 4cX. Gong, W. Ma, J. C. Ostrowski, K. Bechgaard, G. C. Bazan, A. J. Heeger, S. Xiao, D. Moses, Adv. Funct. Mater. 2004, 14, 393;
- 4dH. D. Burrows, J. Seixas de Melo, M. Forster, R. Güntner, U. Scherf, A. P. Monkman, S. Navaratnam, Chem. Phys. Lett. 2004, 385, 105.
- 5
- 5aD. Beljonne, G. Pourtois, C. Silva, E. Hennebicq, L. M. Herz, R. H. Friend, G. D. Scholes, S. Setayesh, K. Müllen, J. L. Brédas, Proc. Natl. Acad. Sci. USA 2002, 99, 10982;
- 5bS. Asaoka, N. Takeda, T. Iyoda, A. R. Cook, J. R. Miller, J. Am. Chem. Soc. 2008, 130, 11912;
- 5cM. E. El-Khouly, Y. Chen, X. Zhuang, S. Fukuzumi, J. Am. Chem. Soc. 2009, 131, 6370;
- 5dY. Shibano, H. Imahori, P. Sreearunothai, A. R. Cook, J. R. Miller, J. Phys. Chem. Lett. 2010, 1, 1492–1496.
- 6
- 6aK. Nomura, N. Yamamoto, R. Ito, M. Fujiki, Y. Geerts, Macromolecules 2008, 41, 4245;
- 6bS. Kuwabara, N. Yamamoto, P. M. V. Sharma, K. Takamizu, M. Fujiki, Y. Geerts, K. Nomura, Macromolecules 2011, 44, 3705;
- 6cM. M. Abdellatif, K. Nomura, ACS Macro Lett. 2012, 1, 423;
- 6dK. Takamizu, A. Inagaki, K. Nomura, ACS Macro Lett. 2013, 2, 980;
- 6eK. Nomura, T. Haque, T. Onuma, F. Hajjaj, M. S. Asano, A. Inagaki, Macromolecules 2013, 46, 9563;
- 6fK. Nomura, T. Haque, T. Miwata, A. Inagaki, K. Takamizu, Polym. Chem. 2015, 6, 380;
- 6gM. Asano, D. Kagota, H. Tahmina, M. Koinuma, A. Inagaki, K. Nomura, Macromolecules 2015, 48, 6233.
- 7For the one-pot synthesis of end-functionalized PFVs, see:
- 7aT. Miyashita, K. Nomura, Macromolecules 2016, 49, 518;
- 7bT. Miyashita, A. Inagaki, K. Nomura, J. Jpn. Pet. Inst. 2016, 59, 197.
- 8
- 8aK. Nomura, H. Morimoto, Y. Imanishi, Z. Ramhani, Y. Geerts, J. Polym. Sci. Part A 2001, 39, 2463;
- 8bN. Yamamoto, R. Ito, Y. Geerts, K. Nomura, Macromolecules 2009, 42, 5104.
- 9For the synthesis of high-molecular-weight poly(2,5-dialkyl-1,4-phenylene vinylene)s (PPVs), see: K. Nomura, Y. Miyamoto, H. Morimoto, Y. Geerts, J. Polym. Sci. Part A 2005, 43, 6166.
- 10For selected reviews on ADMET polymerization, see:
- 10aE. B. Berda, K. B. Wagener in Polymer Science: A Comprehensive Reference, Vol. 5 (Eds.: ), Elsevier BV, Amsterdam, 2012, pp. 195–216;
10.1016/B978-0-444-53349-4.00139-4 Google Scholar
- 10bE. B. Berda, K. B. Wagener in Synthesis of Polymers; New Structures and Methods (Eds.: ), Wiley-VCH, Weinheim, 2012, pp. 587–600;
- 10cP. Atallah, K. B. Wagener, M. D. Schulz, Macromolecules 2013, 46, 4735;
- 10dM. D. Shultz, K. B. Wagener in Handbook of Metathesis, Vol. 3 (Eds.: ), Wiley-VCH, Weinheim, 2015, pp. 313–355;
10.1002/9783527674107.ch39 Google Scholar
- 10eT. Haque, K. Nomura, Catalysts 2015, 5, 500.
- 11For the synthesis of poly(arylene vinylene)s by ADMET polymerization, see:
- 11aH. Weychardt, H. Plenio, Organometallics 2008, 27, 1479;
- 11bY. Qin, M. A. Hillmyer, Macromolecules 2009, 42, 6429;
- 11cJ. C. Speros, B. D. Paulsen, S. P. White, Y. Wu, E. A. Jackson, B. S. Slowinski, C. D. Frisbie, M. A. Hillmyer, Macromolecules 2012, 45, 2190;
- 11dJ. C. Speros, H. Martinez, B. D. Paulsen, S. P. White, A. D. Bonifas, P. C. Goff, C. D. Frisbie, M. A. Hillmyer, Macromolecules 2013, 46, 5184.
- 12For examples of the end functionalization of ROMP polymers and their application for further grafting, see: K. Nomura, M. M. Abdellatif, Polymer 2010, 51, 1861.
- 13For examples of the synthesis of end-functionalized oligo(2,5-dialkoxy-1,4-phenylene vinylene)s by combined olefin metathesis and Wittig coupling, see:
- 13aM. M. Abdellatif, K. Nomura, Org. Lett. 2013, 15, 1618;
- 13bS. Yorsaeng, Y. Kato, K. Tsutsumi, A. Inagaki, B. Kitiyanan, M. Fujiki, K. Nomura, Chem. Eur. J. 2015, 21, 16764.
- 14These results were partly presented at the International Symposium on Catalysis and Fine Chemicals 2016 (November 2016, Taipei, Taiwan).
- 15H. J. Oskam, H. H. Fox, K. B. Yap, H. D. McConville, R. O'Dell, B. J. Lichtenstein, R. R. Schrock, J. Organomet. Chem. 1993, 459, 185.
- 16Detailed experimental procedures, selected NMR spectra and GPC traces, and additional UV/Vis and fluorescence spectra are given in the Supporting Information.
- 17As reported previously,[6–12] the calibration with polystyrene standards overestimates the molecular weight averages of rigid conjugated polymers.[8a] The GPC curves versus structurally similar soluble PPP [poly(para-phenylene)] standards were thus recorded, and the Mn values of rod-like polymers measured versus polymer standards are overestimated by a factor of 1.6.[8a] For a related article, see: D. Marsitzky, T. Brand, Y. Geerts, M. Klapper, K. Müllen, Macromol. Rapid Commun. 1998, 19, 385.
10.1002/(SICI)1521-3927(19980701)19:7<385::AID-MARC385>3.0.CO;2-X CAS Web of Science® Google Scholar
- 18For reports on the charge transfer in ferrocene-containing oligo/poly(thiophene)s, see:
- 18aY. Zhu, M. O. Wolf, J. Am. Chem. Soc. 2000, 122, 10121;
- 18bK. Naka, T. Uemura, Y. Chujo, Macromolecules 2000, 33, 6965;
- 18cT. Li, M. D. Curtis, A. H. Francis, Macromolecules 2002, 35, 4628.
- 19
- 19aP. Anuragudom, S. S. Newaz, S. Phanichphant, T. R. Lee, Macromolecules 2006, 39, 3494;
- 19bQ. Liu, W. Liu, B. Yao, H. Tian, Z. Xie, Y. Geng, F. Wang, Macromolecules 2007, 40, 1851.
- 20In Ref. [6b], we assumed that the unique fluorescence spectra of PFV containing 3T at both chain ends (PFV-3T) could be due to an energy transfer from the PFV conjugated main chain to the oligo(thiophene) moieties [and not due to aggregation of rather planar oligo(thiophene) moieties] because 1) the intensities depended on the PFV chain length (conjugation repeat units), 2) no clear solvent, temperature, or excitation wavelength dependence was observed, and 3) PFV-3T has a longer fluorescence lifetime (λem=530–609 nm in THF) than the other derivatives. Detailed studies including analysis by time-resolved fluorescence spectroscopy are in progress.