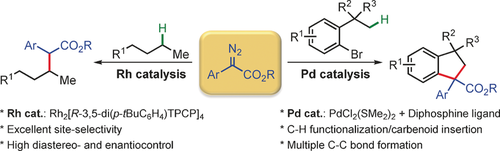
Highlight
Functionalization of Unactivated C(sp3)−H Bonds Using Metal-Carbene Insertion Reactions
Dr. Miguel Peña-López,
Prof. Dr. Matthias Beller,
Corresponding Author
Dr. Miguel Peña-López
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Matthias Beller
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorDr. Miguel Peña-López,
Prof. Dr. Matthias Beller,
Corresponding Author
Dr. Miguel Peña-López
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Matthias Beller
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorGraphical Abstract
References
- 1
- 1aM. P. Doyle, M. A. McKervey, T. Ye, Modern Catalytic Methods for Organic Synthesis with Diazo Compounds, Wiley, New York, 1998, chap. 4 and 5;
- 1bS.-F. Zhu, Q.-L. Zhou, Nat. Sci. Rev. 2014, 1, 580–603;
- 1cX. Zhao, Y. Zhang, J. Wang, Chem. Commun. 2012, 48, 10162–10173;
- 1dY. Zhang, J. Wang, Eur. J. Org. Chem. 2011, 1015–1026;
- 1eA. G. H. Wee, Curr. Org. Synth. 2006, 3, 499–555.
- 2H. M. L. Davies, R. E. J. Beckwith, Chem. Rev. 2003, 103, 2861–2903.
- 3
- 3aL. Liu, J. Zhang, Chem. Soc. Rev. 2016, 45, 506–516;
- 3bA. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire, M. A. McKervey, Chem. Rev. 2015, 115, 9981–10080;
- 3cY. Zhang, J. Wang, Chem. Commun. 2009, 5350–5361;
- 3dH. M. L. Davies, J. R. Denton, Chem. Soc. Rev. 2009, 38, 3061–3071;
- 3eZ. Zhang, J. Wang, Tetrahedron 2008, 64, 6577–6605;
- 3fT. Ye, M. A. McKervey, Chem. Rev. 1994, 94, 1091–1160;
- 3gA. Padwa, D. J. Austin, Angew. Chem. Int. Ed. Engl. 1994, 33, 1797–1815; Angew. Chem. 1994, 106, 1881–1899.
- 4For a general review on Wolff rearrangement, see:
- 4aW. Kirmse, Eur. J. Org. Chem. 2002, 2193–2256; For a review on ketenes as reactive intermediates, see:
- 4bA. D. Allen, T. T. Tidwell, Chem. Rev. 2013, 113, 7287–7342.
- 5
- 5aH. Pellissier, Tetrahedron 2008, 64, 7041–7095;
- 5bH. M. L. Davies, E. G. Antoulinakis, Org. React. 2004, 57, 1–326;
- 5cH. Lebel, J.-F. Marcoux, C. Molinaro, A. B. Charette, Chem. Rev. 2003, 103, 977–1050.
- 6
- 6aS. E. Reisman, R. R. Nani, S. Levin, Synlett 2011, 2437–2442;
- 6bO. A. McNamara, A. R. Maguire, Tetrahedron 2011, 67, 9–40.
- 7
- 7aA. Caballero, M. M. Díaz-Requejo, M. R. Fructos, A. Olmos, J. Urbano, P. J. Pérez, Dalton Trans. 2015, 44, 20295–20307;
- 7bB. Xu, M.-L. Li, X.-D. Zuo, S.-F. Zhu, Q.-L. Zhou, J. Am. Chem. Soc. 2015, 137, 8700–8703;
- 7cS. Jia, D. Xing, D. Zhang, W. Hu, Angew. Chem. Int. Ed. 2014, 53, 13098–13101; Angew. Chem. 2014, 126, 13314–13317.
- 8
- 8aH. Daorui, C. Shenxin, Z. Xueyan, Y. Qiuhan, Y. Qizheng, D. Zhenya, Austin J. Anal. Pharm. Chem. 2016, 3, 1064;
- 8bD. Gillingham, N. Fei, Chem. Soc. Rev. 2013, 42, 4918–4931;
- 8cS.-F. Zhu, Q.-L. Zhou, Acc. Chem. Res. 2012, 45, 1365–1377.
- 9
- 9aJ. V. Santiago, A. H. L. Machado, Beilstein J. Org. Chem. 2016, 12, 882–902;
- 9bH. M. L. Davies, D. Morton, Chem. Soc. Rev. 2011, 40, 1857–1869;
- 9cM. P. Doyle, R. Duffy, M. Ratnikov, L. Zhou, Chem. Rev. 2010, 110, 704–724;
- 9dH. M. L. Davies, A. R. Dick, Top. Curr. Chem. 2010, 292, 303–345;
- 9eC. N. Slattery, A. Ford, A. R. Maguire, Tetrahedron 2010, 66, 6681–6705;
- 9fH. M. L. Davies, J. R. Manning, Nature 2008, 451, 417–424.
- 10H. M. L. Davies, T. Hansen, J. Am. Chem. Soc. 1997, 119, 9075–9076.
- 11K. Liao, S. Negretti, D. G. Musaev, J. Bacsa, H. M. L. Davies, Nature 2016, 533, 230–234.
- 12
- 12aC. M. Quin, H. M. L. Davies, J. Am. Chem. Soc. 2014, 136, 9792–9796;
- 12bD. M. Guptill, H. M. L. Davies, J. Am. Chem. Soc. 2014, 136, 17718–17721.
- 13
- 13aB. Wang, D. Qiu, Y. Zhang, J. Wang, Beilstein J. Org. Chem. 2016, 12, 796–804;
- 13bF. Hu, Y. Xia, C. Ma, Y. Zhang, J. Wang, Chem. Commun. 2015, 51, 7986–7995;
- 13cQ. Xiao, Y. Zhang, J. Wang, Acc. Chem. Res. 2013, 46, 236–247;
- 13dY. Xia, Y. Zhang, J. Wang, ACS Catal. 2013, 3, 2586–2598.
- 14A. Gutiérrez-Bonet, F. Juliá-Hernández, B. de Luis, R. Martin, J. Am. Chem. Soc. 2016, 138, 6384–6387.