Lessons from 1,3-Hydride Shifts in Sesquiterpene Cyclizations
Graphical Abstract
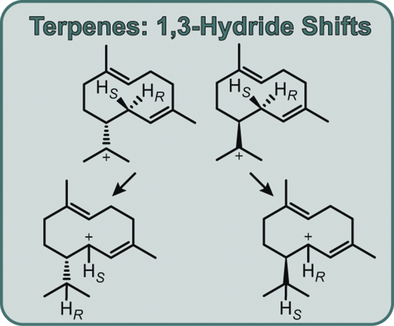
Jump ahead: Incubation experiments with (11-13C,1,1-2H2)FPP and stereospecifically labelled (1R)- and (1S)-(1-2H)FPP enabled assignment of the previously unknown absolute configurations of three bacterial sesquiterpenes from 1,3-hydride shifts, and provided first experimental evidence for a cyclization mechanism to amorphanes that was previously proposed on the basis of quantum chemical calculations.
Abstract
Stereospecifically labelled precursors were subjected to conversion by seven bacterial sesquiterpene cyclases to investigate the stereochemistry of their initial 1,10-cyclisation-1,3-hydride shift cascades. Enzymes with products of known absolute configuration showed a coherent stereochemical course, except for (−)-α-amorphene synthase, for which the obtained results are better explained by an initial 1,6-cyclisation. The link between the absolute configuration of the product and the stereochemical course of the 1,3-hydride shifts enabled assignment of the absolute configurations of three enzyme products, which were confirmed independently through the absolute configuration of the common byproduct germacrene D-4-ol.