Direct and Versatile Synthesis of Red-Shifted Azobenzenes
Mickel J. Hansen
Centre for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands
Search for more papers by this authorMichael M. Lerch
Centre for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands
Search for more papers by this authorCorresponding Author
Dr. Wiktor Szymanski
Centre for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands
Department of Radiology, University of Groningen, University Medical Center Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands
Search for more papers by this authorCorresponding Author
Prof. Dr. Ben L. Feringa
Centre for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands
Search for more papers by this authorMickel J. Hansen
Centre for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands
Search for more papers by this authorMichael M. Lerch
Centre for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands
Search for more papers by this authorCorresponding Author
Dr. Wiktor Szymanski
Centre for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands
Department of Radiology, University of Groningen, University Medical Center Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands
Search for more papers by this authorCorresponding Author
Prof. Dr. Ben L. Feringa
Centre for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands
Search for more papers by this authorGraphical Abstract
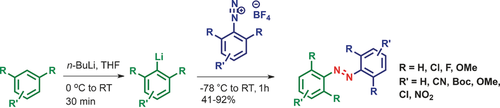
Red-shifted tetra-ortho-substituted azobenzenes were synthesized in a rapid manner with high functional group tolerance (see picture). The privileged tetra-ortho-methoxy, -chloro, and -fluoro azobenzenes become readily accessible, which paves the way for future applications of red-shifted azobenzenes in material and biological sciences.
Abstract
A straightforward synthesis of azobenzenes with bathochromically-shifted absorption bands is presented. It employs an ortho-lithiation of aromatic substrates, followed by a coupling reaction with aryldiazonium salts. The products are obtained with good to excellent yields after simple purification. Moreover, with the presented methodology, a structurally diverse panel of different azobenzenes, including unsymmetric tetra-ortho-substituted ones, can be readily obtained, which paves the way for future development of red-light-addressable azobenzene derivatives for in vivo application.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201607529-sup-0001-misc_information.pdf6.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Molecular Switches (Eds.: ), Wiley-VCH, Weinheim, 2011.
- 2M.-M. Russew, S. Hecht, Adv. Mater. 2010, 22, 3348–3360.
- 3W. Szymański, J. M. Beierle, H. A. V. Kistemaker, W. A. Velema, B. L. Feringa, Chem. Rev. 2013, 113, 6114–6178.
- 4M. Dong, A. Babalhavaeji, S. Samanta, A. A. Beharry, G. A. Woolley, Acc. Chem. Res. 2015, 48, 2662–2670.
- 5C. Brieke, F. Rohrbach, A. Gottschalk, G. Mayer, A. Heckel, Angew. Chem. Int. Ed. 2012, 51, 8446–8476; Angew. Chem. 2012, 124, 8572–8604.
- 6S. Samanta, C. Qin, A. J. Lough, G. A. Woolley, Angew. Chem. Int. Ed. 2012, 51, 6452–6455; Angew. Chem. 2012, 124, 6558–6561.
- 7A. A. Beharry, T. Chen, M. S. Al-Abdul-Wahid, S. Samanta, K. Davidov, O. Sadovski, A. M. Ali, S. B. Chen, R. S. Prosser, H. S. Chan, et al., Biochemistry 2012, 51, 6421–6431.
- 8T. E. Schrader, T. Cordes, W. J. Schreier, F. O. Koller, S.-L. Dong, L. Moroder, W. Zinth, J. Phys. Chem. B 2011, 115, 5219–5226.
- 9W. A. Velema, W. Szymanski, B. L. Feringa, J. Am. Chem. Soc. 2014, 136, 2178–2191.
- 10J. Broichhagen, J. A. Frank, D. Trauner, Acc. Chem. Res. 2015, 48, 1947–1960.
- 11M. J. Hansen, W. A. Velema, G. de Bruin, H. S. Overkleeft, W. Szymanski, B. L. Feringa, ChemBioChem 2014, 15, 2053–2057.
- 12M. Borowiak, W. Nahaboo, M. Reynders, K. Nekolla, P. Jalinot, J. Hasserodt, M. Rehberg, M. Delattre, S. Zahler, A. Vollmar, et al., Cell 2015, 162, 403–411.
- 13M. Volgraf, P. Gorostiza, R. Numano, R. H. Kramer, E. Y. Isacoff, D. Trauner, Nat. Chem. Biol. 2006, 2, 47–52.
- 14W. A. Velema, J. P. van der Berg, M. J. Hansen, W. Szymanski, A. J. M. Driessen, B. L. Feringa, Nat. Chem. 2013, 5, 924–928.
- 15A. A. Beharry, O. Sadovski, G. A. Woolley, J. Am. Chem. Soc. 2011, 133, 19684–19687.
- 16E. Merino, Chem. Soc. Rev. 2011, 40, 3835–3853.
- 17D. Bléger, S. Hecht, Angew. Chem. Int. Ed. 2015, 54, 11338–11349; Angew. Chem. 2015, 127, 11494–11506.
- 18Y. Yang, R. P. Hughes, I. Aprahamian, J. Am. Chem. Soc. 2014, 136, 13190–13193.
- 19R. Siewertsen, H. Neumann, B. Buchheim-Stehn, R. Herges, C. Näther, F. Renth, F. Temps, J. Am. Chem. Soc. 2009, 131, 15594–15595.
- 20R. Weissleder, V. Ntziachristos, Nat. Med. 2003, 9, 123–128.
- 21D. Bléger, J. Schwarz, A. M. Brouwer, S. Hecht, J. Am. Chem. Soc. 2012, 134, 20597–20600.
- 22S. Samanta, A. Babalhavaeji, M. Dong, G. A. Woolley, Angew. Chem. Int. Ed. 2013, 52, 14127–14130; Angew. Chem. 2013, 125, 14377–14380.
- 23S. Samanta, A. A. Beharry, O. Sadovski, T. M. McCormick, A. Babalhavaeji, V. Tropepe, G. A. Woolley, J. Am. Chem. Soc. 2013, 135, 9777–9784.
- 24M. Dong, A. Babalhavaeji, M. J. Hansen, L. Kalman, A. Woolley, Chem. Commun. 2015, 51, 12981–12984.
- 25A. Rullo, A. Reiner, A. Reiter, D. Trauner, E. Y. Isacoff, G. A. Woolley, Chem. Commun. 2014, 50, 14613–14615.
- 26D. B. Konrad, J. A. Frank, D. Trauner, Chem. Eur. J. 2016, 22, 4364–4368.
- 27S. Okumura, C. H. Lin, Y. Takeda, S. Minakata, J. Org. Chem. 2013, 78, 12090–12105.
- 28M. Giannerini, M. Fañanás-Mastral, B. L. Feringa, Nat. Chem. 2013, 5, 667–672.
- 29V. Hornillos, M. Giannerini, C. Vila, M. Fañanás-Mastral, B. L. Feringa, Chem. Sci. 2015, 6, 1394–1398.
- 30V. Snieckus, Chem. Rev. 1990, 90, 879–933.
- 31K. Groom, S. M. S. Hussain, J. Morin, C. Nilewski, T. Rantanen, V. Snieckus, Org. Lett. 2014, 16, 2378–2381.
- 32D. W. Slocum, T. K. Reinscheld, C. B. White, M. D. Timmons, P. A. Shelton, M. G. Slocum, R. D. Sandlin, E. G. Holland, D. Kusmic, J. A. Jennings, et al., Organometallics 2013, 32, 1674–1686.
- 33D. Y. Curtin, J. A. Ursprung, J. Org. Chem. 1956, 21, 1221–1225.
- 34P. A. S. Smith, C. D. Rowe, L. B. Bruner, J. Org. Chem. 1969, 34, 3430–3433.
- 35B. Haag, Z. Peng, P. Knochel, Org. Lett. 2009, 11, 4270–4273.
- 36T. Wendler, C. Schütt, C. Näther, R. Herges, J. Org. Chem. 2012, 77, 3284–3287.
- 37J. T. Manka, V. C. McKenzie, P. Kaszynski, J. Org. Chem. 2004, 69, 1967–1971.
- 38S. Mahouche-Chergui, S. Gam-Derouich, C. Mangeney, M. M. Chehimi, Chem. Soc. Rev. 2011, 40, 4143–4166.
- 39A. M. Roe, R. A. Burton, G. L. Willey, M. W. Baines, A. C. Rasmussen, J. Med. Chem. 1968, 11, 814–819.