Catalytic Asymmetric C −H Functionalization under Photoredox Conditions by Radical Translocation and Stereocontrolled Alkene Addition
−H Functionalization under Photoredox Conditions by Radical Translocation and Stereocontrolled Alkene Addition
Chuanyong Wang
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg, Germany
Search for more papers by this authorDr. Klaus Harms
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Eric Meggers
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg, Germany
Search for more papers by this authorChuanyong Wang
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg, Germany
Search for more papers by this authorDr. Klaus Harms
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Eric Meggers
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg, Germany
Search for more papers by this authorGraphical Abstract
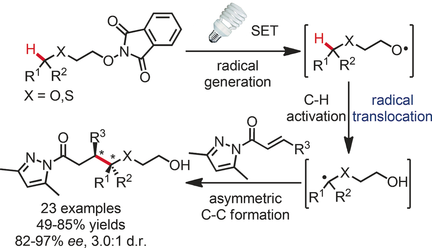
The light combo: Through radical translocation, a photoredox-mediated C(sp3)−H activation was combined with asymmetric catalysis. Upon irradiation with visible light, α,β-unsaturated N-acylpyrazoles react with N-alkoxyphthalimides in the presence of a rhodium-based chiral Lewis acid catalyst and the photosensitizer fac-[Ir(ppy)3] to provide a C−C bond-formation product with high enantioselectivity and, where applicable, with some diastereoselectivity.
Abstract
This work demonstrates how photoredox-mediated C(sp3)−H activation through radical translocation can be combined with asymmetric catalysis. Upon irradiation with visible light, α,β-unsaturated N-acylpyrazoles react with N-alkoxyphthalimides in the presence of a rhodium-based chiral Lewis acid catalyst and the photosensitizer fac-[Ir(ppy)3] to provide a C−C bond-formation product with high enantioselectivity (up to 97 % ee) and, where applicable, with some diastereoselectivity (3.0:1 d.r.). Mechanistically, the synthetic strategy exploits a radical translocation (1,5-hydrogen transfer) from an oxygen-centered to a carbon-centered radical with a subsequent stereocontrolled radical alkene addition.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201607305-sup-0001-misc_information.pdf5.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on different strategies of catalytic asymmetric C−H functionalization, see:
- 1aH. M. L. Davies, J. R. Manning, Nature 2008, 451, 417–424;
- 1bR. Giri, B.-F. Shi, K. M. Engle, N. Maugel, J.-Q. Yu, Chem. Soc. Rev. 2009, 38, 3242–3272;
- 1cM. P. Doyle, R. Duffy, M. Ratnikov, L. Zhou, Chem. Rev. 2010, 110, 704–724;
- 1dS. A. Girard, T. Knauber, C.-J. Li, Angew. Chem. Int. Ed. 2014, 53, 74–100; Angew. Chem. 2014, 126, 76–103;
- 1eC. Zheng, S.-L. You, RSC Adv. 2014, 4, 6173–6214.
- 2
- 2aM. E. Wolff, Chem. Rev. 1963, 63, 55–64;
- 2bG. Majetich, K. Wheless, Tetrahedron 1995, 51, 7095–7129;
- 2cJ. Robertson, J. Pillai, R. K. Lush, Chem. Soc. Rev. 2001, 30, 94–103;
- 2dA. Gansäuer, T. Lauterbach, S. Narayan, Angew. Chem. Int. Ed. 2003, 42, 5556–5573; Angew. Chem. 2003, 115, 5714–5731;
- 2eŽ. Čeković, J. Serb. Chem. Soc. 2005, 70, 287–318;
- 2fF. Dénès, F. Beaufils, P. Renaud, Synlett 2008, 2389–2399;
- 2gJ. Sperry, Y.-C. Liu, M. A. Brimble, Org. Biomol. Chem. 2010, 8, 29–38;
- 2hM. C. Haibach, D. Seidel, Angew. Chem. Int. Ed. 2014, 53, 5010–5036; Angew. Chem. 2014, 126, 5110–5137;
- 2iM. Nechab, S. Mondal, M. P. Bertrand, Chem. Eur. J. 2014, 20, 16034–16059.
- 3
- 3aC. K. Prier, D. A. Rankic, D. W. C. MacMillan, Chem. Rev. 2013, 113, 5322–5363;
- 3bD. P. Hari, B. König, Angew. Chem. Int. Ed. 2013, 52, 4734–4743; Angew. Chem. 2013, 125, 4832–4842;
- 3cF. Dénès, M. Pichowicz, G. Povie, P. Renaud, Chem. Rev. 2014, 114, 2587–2693;
- 3dJ. W. Beatty, C. R. J. Stephenson, Acc. Chem. Res. 2015, 48, 1474–1484;
- 3eJ.-R. Chen, X.-Q. Hu, L.-Q. Lu, W.-J. Xiao, Chem. Soc. Rev. 2016, 45, 2044–2056.
- 4J. Zhang, Y. Li, F. Zhang, C. Hu, Y. Chen, Angew. Chem. Int. Ed. 2016, 55, 1872–1875; Angew. Chem. 2016, 128, 1904–1907.
- 5For photoinduced electron-transfer-promoted redox fragmentation of N-alkoxyphthalimides, see also: M. Zlotorzynska, G. M. Sammis, Org. Lett. 2011, 13, 6264–6267.
- 6For the first report on N-alkoxyphthalimides for the generation of alkoxyl radicals, see: S. Kim, T. A. Lee, Y. Song, Synlett 1998, 471–472.
- 7
- 7aD. P. Curran, D. Kim, H. T. Liu, W. Shen, J. Am. Chem. Soc. 1988, 110, 5900–5902;
- 7bD. P. Curran, W. Shen, J. Am. Chem. Soc. 1993, 115, 6051–6059.
- 8For a review on radical translocations initiated by alkoxyl radicals, see: Ž. Čeković, Tetrahedron 2003, 59, 8073–8090.
- 9For seminal work on chiral Lewis acid catalysis for conjugate radical additions, see:
- 9aM. P. Sibi, J. Ji, J. H. Wu, S. Gürtler, N. A. Porter, J. Am. Chem. Soc. 1996, 118, 9200–9201;
- 9bM. P. Sibi, J. Ji, J. Org. Chem. 1997, 62, 3800–3801.
- 10D. P. Curran, N. A. Porter, B. Giese, Stereochemistry of Radical Reactions, VCH, Weinheim, 1996.
- 11For reviews on enantioselective radical reactions, see:
- 11aM. P. Sibi, S. Manyem, J. Zimmerman, Chem. Rev. 2003, 103, 3263–3296;
- 11bJ. Zimmerman, M. P. Sibi, Top. Curr. Chem. 2006, 263, 107–162.
- 12For a recent review on the catalysis of radical reactions, including asymmetric catalysis, see: A. Studer, D. P. Curran, Angew. Chem. Int. Ed. 2016, 55, 58–102; Angew. Chem. 2016, 128, 58–106.
- 13
- 13aH. Huo, X. Shen, C. Wang, L. Zhang, P. Röse, L.-A. Chen, K. Harms, M. Marsch, G. Hilt, E. Meggers, Nature 2014, 515, 100–103;
- 13bH. Huo, C. Wang, K. Harms, E. Meggers, J. Am. Chem. Soc. 2015, 137, 9551–9554;
- 13cC. Wang, J. Qin, X. Shen, R. Riedel, K. Harms, E. Meggers, Angew. Chem. Int. Ed. 2016, 55, 685–688; Angew. Chem. 2016, 128, 695–698.
- 14C. Wang, L.-A. Chen, H. Huo, X. Shen, K. Harms, L. Gong, E. Meggers, Chem. Sci. 2015, 6, 1094–1100.
- 15For dual photoredox catalysis, see:
- 15aM. N. Hopkinson, B. Sahoo, J.-L. Li, F. Glorius, Chem. Eur. J. 2014, 20, 3874–3886;
- 15bK. L. Skubi, T. R. Blum, T. P. Yoon, Chem. Rev. 2016, 116, 10035–10074;
- 15cY.-Y. Gui, L. Sun, Z.-P. Lu, D.-G. Yu, Org. Chem. Front. 2016, 3, 522–526.
- 16J. Ma, X. Shen, K. Harms, E. Meggers, Dalton Trans. 2016, 45, 8320–8323.
- 17For a recent example in which we combined this rhodium-based Lewis acid catalyst with an additional photoredox sensitizer, see: H. Huo, K. Harms, E. Meggers, J. Am. Chem. Soc. 2016, 138, 6936–6939.
- 18See the Supporting Information for additional experiments regarding the dependence of enantioselectivity with the light source and intensity.
- 19For a related observation and interpretation, see Ref. [4]. For photoinduced electron transfer by direct excitation of Hantzsch ester with visible light, see also:
- 19aX.-Q. Zhu, Y.-C. Liu, J.-P. Cheng, J. Org. Chem. 1999, 64, 8980–8981;
- 19bJ. Jung, J. Kim, G. Park, Y.-M. You, E. J. Cho, Adv. Synth. Catal. 2016, 358, 74–80;
- 19cW. Chen, H. Tao, W. Huang, G. Qang, S. Li, X. Cheng, G. Li, Chem. Eur. J. 2016, 22, 9546–9550.
- 20
- 20aX.-Q. Zhu, H.-R. Li, Q. Li, T. Ai, J.-Y. Lu, Y. Yang, J.-P. Cheng, Chem. Eur. J. 2003, 9, 871–880;
- 20bA. Singh, K. Teegardin, M. Kelly, K. S. Prasad, S. Krishnan, J. D. Weaver, J. Organomet. Chem. 2015, 776, 51–59.
- 21For a review on the reactivity of alkoxy radicals, see: J. Hartung, T. Gottwald, K. Špehar, Synthesis 2002, 1469–1498.
- 22For selected recent examples on 1,5-HAT initiated by alkoxyl radicals, see:
- 22aC. G. Francisco, A. J. Herrera, E. Suárez, J. Org. Chem. 2002, 67, 7439–7445;
- 22bH. Zhu, J. G. Wickenden, N. E. Campbell, J. C. T. Leung, K. M. Johnson, G. M. Sammis, Org. Lett. 2009, 11, 2019–2022;
- 22cR. Kundu, Z. T. Ball, Org. Lett. 2010, 12, 2460–2463;
- 22dH. Zhu, J. C. T. Leung, G. M. Sammis, J. Org. Chem. 2015, 80, 965–979.
- 23For the relatively sluggish conversion 1 a+2 h→3 s (Figure 2) we were able to isolate 2-((1-tosylazetidin-3-yl)oxy)ethanol as a side product. This furthermore supports our proposed mechanism and can be explained by a competing undesired reduction of the initial oxygen- or carbon-centered radicals. See the Supporting Information for more details.
- 24M. A. Cismesia, T. P. Yoon, Chem. Sci. 2015, 6, 5426–5434.
- 25CCDC 1492579 (3 r) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.