An Electrophilic Reagent for the Direct Introduction of the SCF2PO(OEt)2 Group to Molecules
Heng-Ying Xiong
Normandie Univ, INSA Rouen, UNIROUEN, CNRS, COBRA (UMR 6014), 76000 Rouen, France
Search for more papers by this authorDr. Alexandre Bayle
Normandie Univ, INSA Rouen, UNIROUEN, CNRS, COBRA (UMR 6014), 76000 Rouen, France
Search for more papers by this authorProf. Xavier Pannecoucke
Normandie Univ, INSA Rouen, UNIROUEN, CNRS, COBRA (UMR 6014), 76000 Rouen, France
Search for more papers by this authorCorresponding Author
Dr. Tatiana Besset
Normandie Univ, INSA Rouen, UNIROUEN, CNRS, COBRA (UMR 6014), 76000 Rouen, France
Search for more papers by this authorHeng-Ying Xiong
Normandie Univ, INSA Rouen, UNIROUEN, CNRS, COBRA (UMR 6014), 76000 Rouen, France
Search for more papers by this authorDr. Alexandre Bayle
Normandie Univ, INSA Rouen, UNIROUEN, CNRS, COBRA (UMR 6014), 76000 Rouen, France
Search for more papers by this authorProf. Xavier Pannecoucke
Normandie Univ, INSA Rouen, UNIROUEN, CNRS, COBRA (UMR 6014), 76000 Rouen, France
Search for more papers by this authorCorresponding Author
Dr. Tatiana Besset
Normandie Univ, INSA Rouen, UNIROUEN, CNRS, COBRA (UMR 6014), 76000 Rouen, France
Search for more papers by this authorGraphical Abstract
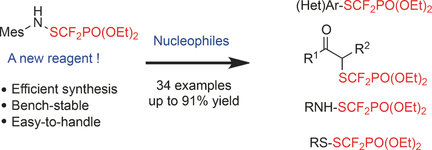
Tool up: A new electrophilic difluoromethylthiolating reagent (MesNHSCF2PO(OEt)2) was treated with various nucleophiles under mild and metal-free conditions, thus offering an efficient and operationally simple tool for the construction of C−SCF2PO(OR)2, N−SCF2PO(OR)2, and S−SCF2PO(OR)2 bonds. The versatility of this method was illustrated through the synthesis of the non-stereoidal anti-inflammatory compound diflumidone.
Abstract
An unprecedented electrophilic difluoromethylthiolating reagent (MesNHSCF2PO(OEt)2) was designed. Under mild and metal-free conditions, this new reagent reacted with various nucleophiles, thus offering an efficient and operationally simple tool for the construction of C−SCF2PO(OR)2, N−SCF2PO(OR)2, and S−SCF2PO(OR)2 bonds. Finally, thanks to this new methodology, the synthesis of the non-stereoidal anti-inflammatory diflumidone was achieved.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201607231-sup-0001-misc_information.pdf5.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok, H. Liu, Chem. Rev. 2014, 114, 2432–2506;
- 1bS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320–330;
- 1cE. A. Ilardi, E. Vitaku, J. T. Njardarson, J. Med. Chem. 2014, 57, 2832–2842;
- 1dE. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly, N. A. Meanwell, J. Med. Chem. 2015, 58, 8315–8359.
- 2
- 2aX.-H. Xu, K. Matsuzaki, N. Shibata, Chem. Rev. 2015, 115, 731–764, and references therein;
- 2bC. Ni, M. Hu, J. Hu, Chem. Rev. 2015, 115, 765–825;
- 2cM.-C. Belhomme, T. Besset, T. Poisson, X. Pannecoucke, Chem. Eur. J. 2015, 21, 12836–12865;
- 2dC. Ni, L. Zhu, J. Hu, Acta Chim. Sin. 2015, 73, 90–115;
- 2eT. Besset, T. Poisson, X. Pannecoucke, Eur. J. Org. Chem. 2015, 2765–2789;
- 2fT. Besset, T. Poisson, X. Pannecoucke, Chem. Eur. J. 2014, 20, 16830–16845;
- 2gH. Egami, M. Sodeoka, Angew. Chem. Int. Ed. 2014, 53, 8294–8308; Angew. Chem. 2014, 126, 8434–8449;
- 2hT. Liang, C. N. Neumann, T. Ritter, Angew. Chem. Int. Ed. 2013, 52, 8214–8264; Angew. Chem. 2013, 125, 8372–8423;
- 2iG. Landelle, A. Panossian, S. Pazenok, J.-P. Vors, F. R. Leroux, Beilstein J. Org. Chem. 2013, 9, 2476–2536;
- 2jT. Furuya, A. S. Kamlet, T. Ritter, Nature 2011, 473, 470–477;
- 2kB. Manteau, S. Pazenok, J. P. Vors, F. R. Leroux, J. Fluorine Chem. 2010, 131, 140–158;
- 2lT. Besset, P. Jubault, X. Pannecoucke, T. Poisson, Org. Chem. Front. 2016, 3, 1004–1010.
- 3
- 3aD. O'Hagan, Chem. Soc. Rev. 2008, 37, 308–319;
- 3bD. O'Hagan, H. S. Rzepa, Chem. Commun. 1997, 645–652;
- 3cK. Uneyama, Organofluorine Chemistry, Blackwell Publishing Ltd., Oxford, 2006.
10.1002/9780470988589 Google Scholar
- 4For selected reviews, see:
- 4aF. Toulgoat, S. Alazet, T. Billard, Eur. J. Org. Chem. 2014, 2415–2428;
- 4bS. Barata-Vallejo, S. Bonesi, A. Postigo, Org. Biomol. Chem. 2016, 14, 7150–7182; for selected examples, see:
- 4cS. Alazet, L. Zimmer, T. Billard, Chem. Eur. J. 2014, 20, 8589–8593;
- 4dB. Exner, B. Bayarmagnai, F. Jia, L. J. Gooßen, Chem. Eur. J. 2015, 21, 17220–17223;
- 4eM. Hu, J. Rong, W. Miao, C. Ni, Y. Han, J. Hu, Org. Lett. 2014, 16, 2030–2033;
- 4fF. Hu, X.-X. Shao, D.-H. Zu, L. Lu, Q. Shen, Angew. Chem. Int. Ed. 2014, 53, 6105–6109; Angew. Chem. 2014, 126, 6219–6223;
- 4gJ.-B. Liu, X.-H. Xu, Z.-H. Chen, F.-L. Qing, Angew. Chem. Int. Ed. 2015, 54, 897–900; Angew. Chem. 2015, 127, 911–914;
- 4hT. Yang, L. Lu, Q. Shen, Chem. Commun. 2015, 51, 5479–5481;
- 4iH.-Y. Xiong, T. Besset, D. Cahard, X. Pannecoucke, J. Org. Chem. 2015, 80, 4204–4212;
- 4jK.-Y. Ye, X. Zhang, L.-X. Dai, S.-L. You, J. Org. Chem. 2014, 79, 12106–12110;
- 4kZ. Huang, Y.-D. Yang, E. Tokunaga, N. Shibata, Org. Lett. 2015, 17, 1063–1065;
- 4lK. Zhang, J.-B. Liu, F.-L. Qing, Chem. Commun. 2014, 50, 14157–14160;
- 4mR. Pluta, P. Nikolaienko, M. Rueping, Angew. Chem. Int. Ed. 2014, 53, 1650–1653; Angew. Chem. 2014, 126, 1676–1679;
- 4nL. Jiang, J. Qian, W. Yi, G. Lu, C. Cai, W. Zhang, Angew. Chem. Int. Ed. 2015, 54, 14965–14969; Angew. Chem. 2015, 127, 15178–15182;
- 4oY. Yang, L. Xu, S. Yu, X. Liu, Y. Zhang, D. A. Vicic, Chem. Eur. J. 2016, 22, 858–863;
- 4pQ. Wang, Z. Qi, F. Xie, X. Li, Adv. Synth. Catal. 2015, 357, 355–360.
- 5
- 5aB. Bayarmagnai, C. Matheis, K. Jouvin, L. J. Gooßen, Angew. Chem. Int. Ed. 2015, 54, 5753–5756; Angew. Chem. 2015, 127, 5845–5848;
- 5bK. Jouvin, C. Matheis, L. J. Gooßen, Chem. Eur. J. 2015, 21, 14324–14327;
- 5cJ. Wu, Y. Gu, X. Leng, Q. Shen, Angew. Chem. Int. Ed. 2015, 54, 7648–7652; Angew. Chem. 2015, 127, 7758–7762;
- 5dJ. Wu, Y. Liu, C. Lu, Q. Shen, Chem. Sci. 2016, 7, 3757–3762;
- 5eD. Zhu, Y. Gu, L. Lu, Q. Shen, J. Am. Chem. Soc. 2015, 137, 10547–10553;
- 5fS. Arimori, O. Matsubara, M. Takada, M. Shiro, N. Shibata, R. Soc. Open Sci. 2016, 3, 160102.
- 6W. Zhang, J. Zhu, J. Hu, Tetrahedron Lett. 2008, 49, 5006–5008.
- 7C. Matheis, B. Bayarmagnai, K. Jouvin, L. J. Gooßen, Org. Chem. Front. 2016, 3, 949–952.
- 8For selected examples, see
- 8aQ. Glenadel, M. Bordy, S. Alazet, A. Tlili, T. Billard, Asian J. Org. Chem. 2016, 5, 428–433;
- 8bF. Baert, J. Colomb, T. Billard, Angew. Chem. Int. Ed. 2012, 51, 10382–10385; Angew. Chem. 2012, 124, 10528–10531;
- 8cA. Tlili, S. Alazet, Q. Glenadel, T. Billard, Chem. Eur. J. 2016, 22, 10230–10234;
- 8dE. Ismalaj, D. Le Bars, T. Billard, Angew. Chem. Int. Ed. 2016, 55, 4790–4793; Angew. Chem. 2016, 128, 4868–4871.
- 9
- 9aJ.-B. Han, H.-L. Qin, S.-H. Ye, L. Zhi, C.-P. Zhang, J. Org. Chem. 2016, 81, 2506–2512;
- 9bY.-M. Lin, W.-B. Yi, W.-Z. Shen, G.-P. Lu, Org. Lett. 2016, 18, 592–595.
- 10For selected reviews, see:
- 10aA. R. Murphy, J. M. J. Fréchet, Chem. Rev. 2007, 107, 1066–1096;
- 10bC. Shen, P. Zhang, Q. Sun, S. Bai, T. S. A. Hor, X. Liu, Chem. Soc. Rev. 2015, 44, 291–314;
- 10cM. V. Ivanova, A. Bayle, T. Besset, X. Pannecoucke, T. Poisson, Chem. Eur. J. 2016, 22, 10284–10293, and references therein.
- 11
- 11aA. Konno, T. Fuchigami, J. Org. Chem. 1997, 62, 8579–8581;
- 11bT. Lequeux, F. Lebouc, C. Lopin, H. Yang, G. Gouhier, S. R. Piettre, Org. Lett. 2001, 3, 185–188;
- 11cA. Henry-dit-Quesnel, L. Toupet, J.-C. Pommelet, T. Lequeux, Org. Biomol. Chem. 2003, 1, 2486–2491;
- 11dL. Aliouane, S. Chao, L. Brizuela, E. Pfund, O. Cuvillier, L. Jean, P.-Y. Renard, T. Lequeux, Bioorg. Med. Chem. 2014, 22, 4955–4960;
- 11eC. De Schutter, E. Pfund, T. Lequeux, Tetrahedron 2013, 69, 5920–5926.
- 12G. Danoun, B. Bayarmagnai, M. F. Grünberg, L. J. Gooßen, Chem. Sci. 2014, 5, 1312–1316.
- 13H.-Y. Xiong, X. Pannecoucke, T. Besset, Org. Chem. Front. 2016, 3, 620–624.
- 14See the Supporting Information for further details.
- 15
- 15aM. V. Ivanova, A. Bayle, T. Besset, T. Poisson, X. Pannecoucke, Angew. Chem. Int. Ed. 2015, 54, 13406–13410; Angew. Chem. 2015, 127, 13604–13608;
- 15bA. Bayle, C. Cocaud, C. Nicolas, O. R. Martin, T. Poisson, X. Pannecoucke, Eur. J. Org. Chem. 2015, 3787–3792.
- 16The reagent 2 can be stored for three months at room temperature under air without any loss of efficiency (see the Supporting Information).
- 17CCDC 1494845 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 18W. Wu, X. Zhang, F. Liang, S. Cao, Org. Biomol. Chem. 2015, 13, 6992–6999.
- 19
- 19aL. Craine, M. Raban, Chem. Rev. 1989, 89, 689–712.
- 20Unfortunately, when the more nucleophilic aliphatic amines were engaged with 2 in CH2Cl2 in the absence of TFA, no reaction occurred. Indeed, 2 was recovered untouched despite longer reaction time, increased reaction temperature, and the presence or not of another additive such as BF3OEt2, TMSCl in replacement of TFA.
- 21P. Beier, A. V. Alexandrova, M. Zibinsky, G. K. S. Prakash, Tetrahedron 2008, 64, 10977–10985.
- 22
- 22aR. A. Scherrer, M. W. Whitehouse, Antiinflammatory agents: Chemistry and Pharmacology, Vol. 2, Academic Press, New York, 1974;
- 22bR. L. Vigdahl, R. H. Tukey, Biochem. Pharmacol. 1977, 26, 307–311.
- 23
- 23aJ. K. Harrington, J. E. Robertson, D. C. Kvam, R. R. Hamilton, K. T. McGurran, R. J. Trancik, K. F. Swingle, G. G. I. Moore, J. F. Gerster, J. Med. Chem. 1970, 13, 137;
- 23bV. V. Farrar, J. Chem. Soc. 1960, 3058–3062.
- 24
- 24aX. J. Tang, C. S. Thomoson, W. R. Dolbier, Jr., Org. Lett. 2014, 16, 4594–4597.