Sequestration of Proteins by Fatty Acid Coacervates for Their Encapsulation within Vesicles
David Garenne
UMR 1332, biologie et pathologie du fruit, INRA, centre de Bordeaux, 33883 Villenave O'Ornon, France
Search for more papers by this authorDr. Laure Beven
UMR 1332, biologie et pathologie du fruit, INRA, centre de Bordeaux, 33883 Villenave O'Ornon, France
Search for more papers by this authorDr. Laurence Navailles
Université de Bordeaux, Centre de Recherche Paul-Pascal, CNRS, av. A. Schweitzer, 33600 Pessac, France
Search for more papers by this authorProf. Frédéric Nallet
Université de Bordeaux, Centre de Recherche Paul-Pascal, CNRS, av. A. Schweitzer, 33600 Pessac, France
Search for more papers by this authorDr. Erick J. Dufourc
Institute of Chemistry and Biology of Membranes and Nano-objects, UMR 5248, CNRS, université de Bordeaux, Institut polytechnique Bordeaux, 33600 Pessac, France
Search for more papers by this authorCorresponding Author
Dr. Jean-Paul Douliez
UMR 1332, biologie et pathologie du fruit, INRA, centre de Bordeaux, 33883 Villenave O'Ornon, France
Search for more papers by this authorDavid Garenne
UMR 1332, biologie et pathologie du fruit, INRA, centre de Bordeaux, 33883 Villenave O'Ornon, France
Search for more papers by this authorDr. Laure Beven
UMR 1332, biologie et pathologie du fruit, INRA, centre de Bordeaux, 33883 Villenave O'Ornon, France
Search for more papers by this authorDr. Laurence Navailles
Université de Bordeaux, Centre de Recherche Paul-Pascal, CNRS, av. A. Schweitzer, 33600 Pessac, France
Search for more papers by this authorProf. Frédéric Nallet
Université de Bordeaux, Centre de Recherche Paul-Pascal, CNRS, av. A. Schweitzer, 33600 Pessac, France
Search for more papers by this authorDr. Erick J. Dufourc
Institute of Chemistry and Biology of Membranes and Nano-objects, UMR 5248, CNRS, université de Bordeaux, Institut polytechnique Bordeaux, 33600 Pessac, France
Search for more papers by this authorCorresponding Author
Dr. Jean-Paul Douliez
UMR 1332, biologie et pathologie du fruit, INRA, centre de Bordeaux, 33883 Villenave O'Ornon, France
Search for more papers by this authorGraphical Abstract
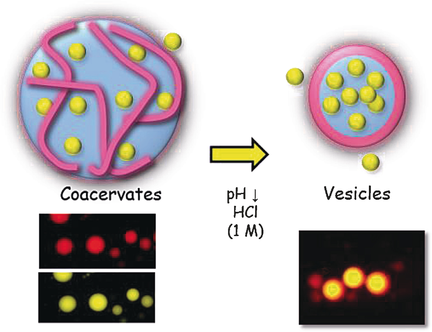
Vesicular microreactors: Fatty acid based, membrane-free coacervates spontaneously sequester proteins and can reversibly form membranous vesicles upon changing the pH value, which leads to protein encapsulation within the vesicles. These micrometric capsules also provide a suitable environment for enzymatic reactions.
Abstract
Encapsulating biological materials in lipid vesicles is of interest for mimicking cells; however, except in some particular cases, such processes do not occur spontaneously. Herein, we developed a simple and robust method for encapsulating proteins in fatty acid vesicles in high yields. Fatty acid based, membrane-free coacervates spontaneously sequester proteins and can reversibly form membranous vesicles upon varying the pH value, the precrowding feature in coacervates allowing for protein encapsulation within vesicles. We then produced enzyme-enriched vesicles and show that enzymatic reactions can occur in these micrometric capsules. This work could be of interest in the field of synthetic biology for building microreactors.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201607117-sup-0001-misc_information.pdf1.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. Fritjof, P. Luigi Luisi, The Systems View of Life, Cambridge University Press, New York, 2014; P. Luigi Luisi, P. Stano, The minimal cell, Springer Science, 2011.
- 2A. J. Dzieciol, S. Mann, Chem. Soc. Rev. 2012, 41, 79–85; S. Mann, Angew. Chem. Int. Ed. 2013, 52, 155–162; Angew. Chem. 2013, 125, 166–173; U. J. Meierhenrich, J.-J. Filippi, C. Meinert, P. Vierling, J. P. Dworkin, Angew. Chem. Int. Ed. 2010, 49, 3738–3750; Angew. Chem. 2010, 122, 3826–3839; Y. Tu, F. Peng, A. Adawy, Y. Men, L. K. E. A. Abdelmohsen, D. A. Wilson, Chem. Rev. 2016, 116, 2023–2078; P. L. Luisi, C. Chiarabelli, P. Stano, Curr. Opin. Chem. Biol. 2014, 22, v–vii; K. Morigaki, P. Walde, Curr. Opin. Colloid Interface Sci. 2007, 12, 75–80; P. Stano, P. L. Luisi, Curr. Opin. Biotechnol. 2013, 24, 633–638; D. Segré, D. Ben-Eli, D. W. Deamer, D. Lancet, Origins Life Evol. Biospheres 2001, 31, 119–145; D. Deamer, Chem. Soc. Rev. 2012, 41, 5375–5379.
- 3T. P. de Souza, A. Fahr, P. L. Luisi, P. Stano, J. Mol. Evol. 2014, 79, 179–192; P. L. Luisi, P. Stano, T. de Souza, Origins Life Evol. Biospheres 2014, 44, 313–317; P.-A. Monnard, D. Deamer, Origins Life Evol. Biospheres 2001, 31, 147–155.
- 4P. Stano, E. D'Aguanno, J. Bolz, A. Fahr, P. L. Luisi, Angew. Chem. Int. Ed. 2013, 52, 13397–13400; Angew. Chem. 2013, 125, 13639–13642.
- 5P. Walde, S. Ichikawa, Biomol. Eng. 2001, 18, 143–177.
- 6Y. Elani, A. Gee, R. V. Law, O. Ces, Chem. Sci. 2013, 4, 3332–3338; Y. Elani, R. V. Law, O. Ces, Nat. Commun. 2014, 5, 5305; P. Torre, C. D. Keating, S. S. Mansy, Langmuir 2014, 30, 5695–5699; V. Noireaux, A. Libchaber, Proc. Natl. Acad. Sci. USA 2004, 101, 17669–17674.
- 7F. Caschera, V. Noireaux, Curr. Opin. Chem. Biol. 2014, 22, 85–91; V. Noireaux, Y. T. Maeda, A. Libchaber, Proc. Natl. Acad. Sci. USA 2011, 108, 3473–3480; S. Pautot, B. J. Frisken, D. A. Weitz, Langmuir 2003, 19, 2870–2879; S. Pautot, B. J. Frisken, D. A. Weitz, Proc. Natl. Acad. Sci. USA 2003, 100, 10718–10721.
- 8C. Martino, S.-H. Kim, L. Horsfall, A. Abbaspourrad, S. J. Rosser, J. Cooper, D. A. Weitz, Angew. Chem. Int. Ed. 2012, 51, 6416–6420; Angew. Chem. 2012, 124, 6522–6526.
- 9A. P. Minton, Curr. Opin. Struct. Biol. 2000, 10, 34–39; D. van Swaay, T. Y. D. Tang, S. Mann, A. de Mello, Angew. Chem. Int. Ed. 2015, 54, 8398–8401; Angew. Chem. 2015, 127, 8518–8521; A. I. Oparin, Origin of Life, Dover Pubns, New York, 1953; S. Koga, D. S. Williams, A. W. Perriman, S. Mann, Nat. Chem. 2011, 3, 720–724; C. D. Keating, Acc. Chem. Res. 2012, 45, 2114–2124; S. Lindhoud, M. M. A. E. Claessens, Soft Matter 2016, 12, 408–413; R. J. Ellis, Trends Biochem. Sci. 2001, 26, 597–604; J. M. Rohwer, P. W. Postma, B. N. Kholodenko, H. V. Westerhoff, Proc. Natl. Acad. Sci. USA 1998, 95, 10547–10552.
- 10M. Wang, Y. Wang, Soft Matter 2014, 10, 7909–7919.
- 11T. Y. D. Tang, C. R. Che Hak, A. J. Thompson, M. K. Kuimova, D. S. Williams, A. W. Perriman, S. Mann, Nat. Chem. 2014, 6, 527–533.
- 12K. Shinoda, S. Friberg, Emulsions and solubilization, Wiley, New York, 1986; T. K. Hodgdon, E. W. Kaler, Curr. Opin. Colloid Interface Sci. 2007, 12, 121–128.
- 13D. Garenne, L. Navailles, F. Nallet, A. Grélard, E. J. Dufourc, J.-P. Douliez, J. Colloid Interface Sci. 2016, 468, 95–102.
- 14J.-P. Douliez, B. Houinsou Houssou, A. L. Fameau, L. Navailles, F. Nallet, A. Grélard, E. J. Dufourc, C. Gaillard, Langmuir 2016, 32, 401–410.
- 15J. M. Gebicki, M. Hicks, Nature 1973, 243, 232–234; M. Hicks, J. M. Gebicki, Chem. Phys. Lipids 1977, 20, 243–252.
- 16C. L. Apel, D. W. Deamer, M. N. Mautner, Biochim. Biophys. Acta Biomembr. 2002, 1559, 1–9.
- 17W. Hargreaves, D. W. Deamer, Biochemistry 1978, 17, 3759–3768.
- 18J. Davis, Biochim. Biophys. Acta Rev. Biomembr. 1983, 737, 117–171.
- 19J.-P. Douliez, B. Houinsou-Houssou, A.-L. Fameau, B. Novales, C. Gaillard, J. Colloid Interface Sci. 2010, 341, 386–389; A.-L. Fameau, B. Houinsou-Houssou, J. Ventureira, B. Novales, L. Navailles, F. Nallet, J.-P. Douliez, Langmuir 2011, 27, 4505–4513.
- 20H. Maeda, S. Matsu-Ura, Y. Yamauchi, H. Ohmori, Chem. Pharm. Bull. 2001, 49, 622–625.
- 21P. Walde, K. Cosentino, H. Engel, P. Stano, ChemBioChem 2010, 11, 848–865; P. Walde, Origins Life Evol. Biospheres 2006, 36, 109–150; J. W. Szostak, D. P. Bartel, P. L. Luisi, Nature 2001, 409, 387–390; R. A. Black, M. C. Blosser, B. L. Stottrup, R. Tavakley, D. W. Deamer, S. L. Keller, Proc. Natl. Acad. Sci. USA 2013, 110, 13272–13276; S. S. Mansy, J. P. Schrum, M. Krishnamurthy, S. Tobe, D. A. Treco, J. W. Szostak, Nature 2008, 454, 122–125.
- 22P. Walde, R. Wick, M. Fresta, A. Mangone, P. L. Luisi, J. Am. Chem. Soc. 1994, 116, 11649–11654; E. Blöchliger, M. Blocher, P. Walde, P. L. Luisi, J. Phys. Chem. 1998, 102, 10383–10390; P. Stano, P. L. Luisi, Chem. Commun. 2010, 46, 3639–3653; I. A. Chen, R. W. Roberts, J. W. Szostak, Science 2004, 305, 1474–1476; M. M. Hanczyc, S. M. Fujikawa, J. W. Szostak, Science 2003, 302, 618–622.
- 23I. Budin, A. Debnath, J. W. Szostak, J. Am. Chem. Soc. 2012, 134, 20812–20819; I. A. Chen, J. W. Szostak, Biophys. J. 2004, 87, 988–998; T. F. Zhu, J. W. Szostak, J. Am. Chem. Soc. 2009, 131, 5705–5713; K. Morigaki, P. Walde, M. Misran, B. H. Robinson, Colloids Surf. A 2003, 213, 37–44.
- 24S. I. Jenkins, C. M. Collins, M. G. Khaledi, Langmuir 2016, 32, 2321–2330; M. G. Khaledi, S. I. Jenkins, S. Liang, Langmuir 2013, 29, 2458–2464.
- 25L. Chiappisi, H. Yalcinkaya, V. K. Gopalakrishnan, M. Gradzielski, T. Zemb, Colloid and Polymer Science 2015, 293, 3131–3143; E. Soussan, S. Cassel, M. Blanzat, I. Rico-Lattes, Angew. Chem. Int. Ed. 2009, 48, 274–288; Angew. Chem. 2009, 121, 280–295; R. O. Brito, E. F. Marques, P. Gomes, S. Falcao, O. Soderman, J. Phys. Chem. B 2006, 110, 18158–18165; D. E. Discher, A. Eisenberg, Science 2002, 297, 967–973; J. Huang, C. Bonduelle, J. Thévenot, S. Lecommandoux, A. Heise, J. Am. Chem. Soc. 2012, 134, 119–122.