Merging Iron Catalysis and Biocatalysis—Iron Carbonyl Complexes as Efficient Hydrogen Autotransfer Catalysts in Dynamic Kinetic Resolutions
Correction(s) for this article
-
Corrigendum: Merging Iron Catalysis and Biocatalysis—Iron Carbonyl Complexes as Efficient Hydrogen Autotransfer Catalysts in Dynamic Kinetic Resolutions
- Volume 56Issue 12Angewandte Chemie International Edition
- pages: 3129-3129
- First Published online: March 10, 2017
Dr. Osama El-Sepelgy
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorNurtalya Alandini
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Magnus Rueping
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
King Abdullah University of Science and Technology (KAUST), KAUST Catalysis Center (KCC), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorDr. Osama El-Sepelgy
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorNurtalya Alandini
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Magnus Rueping
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
King Abdullah University of Science and Technology (KAUST), KAUST Catalysis Center (KCC), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorGraphical Abstract
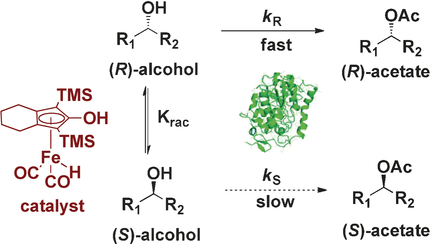
Iron can: Green and inexpensive iron carbonyl complexes are efficient catalysts for the racemization of chiral alcohols by mimicking the action of Fe-hydrogenases. The combination of the iron-based catalyst with lipase provides a platform for the enzymatic resolution of secondary alcohols. TMS=trimethylsilyl.
Abstract
A dual catalytic iron/lipase system has been developed and applied in the dynamic kinetic resolution of benzylic and aliphatic secondary alcohols. A detailed study of the Knölker-type iron complexes demonstrated the hydrogen autotransfer of alcohols to proceed under mild reaction conditions and allowed the combination with the enzymatic resolution. Different racemic alcohols were efficiently converted to chiral acetates in good yields and with excellent enantioselectivities.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201606197-sup-0001-misc_information.pdf1.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1B. Cornils, W. A. Hermann, Applied homogenous catalysis and organometallic compounds, 2nd ed., Wiley-VCH, Weinheim, 2002.
10.1002/9783527618231 Google Scholar
- 2
- 2aC. Bolm, J. Legros, J. Le Paih, L. Zani, Chem. Rev. 2004, 104, 6217;
- 2bB. Plietker, Iron Catalysis in Organic Chemistry: Reactions and applications, 2nd ed., Wiley-VCH, Weinheim, 2008;
10.1002/9783527623273 Google Scholar
- 2cI. Bauer, H.-J. Knölker, Chem. Rev. 2015, 115, 3170.
- 3
- 3aT. C. Nugent, Chiral Amine Synthesis: Methods, Developments and Applications, Wiley-VCH, Weinheim, 2010;
10.1002/9783527629541 Google Scholar
- 3bA. Bartoszewicz, N. Ahlsten, B. Martín-Matute, Chem. Eur. J. 2013, 19, 7274.
- 4
- 4aJ. M. J. Williams, R. J. Parker, C. Neri in Enzyme catalysis in organic synthesis: A comprehensive handbook, 2nd ed (Eds.: ), Wiley-VCH, Weinheim, 2008, pp. 287–312;
- 4bM. Breuer, K. Ditrich, T. Habicher, B. Hauer, M. Kesseler, R. Stürmer, T. Zelinski, Angew. Chem. Int. Ed. 2004, 43, 788; Angew. Chem. 2004, 116, 806;
- 4cA. Ghanem, H. Y. Aboul-Enein, Tetrahedron: Asymmetry 2004, 15, 3331;
- 4dM. D. Romero, J. M. Gomez, B. D. Suelto, A. Garcia-Sanz, N. Baster, Appl. Biochem. Biotechnol. 2011, 165, 1129;
- 4eY. Wang, R. Wang, Q. Li, Z. Zhang, Y. Feng, J. Mol. Catal. B 2009, 56, 142;
- 4fJ. Lee, Y. Oh, Y. K. Choi, E. Choi, K. Kim, J. Park, M.-J. Kim, ACS Catal. 2014, 5, 638.
- 5Y. Ahn, S.-B. Ko, M.-J. Kim, J. Park, Coord. Chem. Rev. 2008, 252, 647.
- 6P. M. Dinh, J. A. Howarth, A. R. Hudnott, J. M. J. Williams, W. Harris, Tetrahedron Lett. 1996, 37, 7623.
- 7For selected pioneering work on ruthenium and enzyme catalyzed DKR, see:
- 7aA. L. E. Larsson, B. A. Persson, J.-E. Bäckvall, Angew. Chem. Int. Ed. Engl. 1997, 36, 1211; Angew. Chem. 1997, 109, 1256;
- 7bB. A. Persson, A. L. E. Larsson, M. Le Ray, J.-E. Bäckvall, J. Am. Chem. Soc. 1999, 121, 1645;
- 7cJ. H. Koh, H. M. Jung, M.-J. Kim, J. Park, Tetrahedron Lett. 1999, 40, 6281;
- 7dD. Lee, E. A. Huh, M.-J. Kim, H. M. Jung, J. H. Koh, J. Park, Org.Lett. 2000, 2, 2377;
- 7eJ. H. Choi, Y. H. Kim, S. H. Nam, S. T. Shin, M.-J. Kim, J. Park, Angew. Chem. Int. Ed. 2002, 41, 2373;
10.1002/1521-3773(20020703)41:13<2373::AID-ANIE2373>3.0.CO;2-7 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 2479;
- 7fB. Martín-Matute, M. Edin, K. Bogár, F. B. Kaynak, J.-E. Bäckvall, J. Am. Chem. Soc. 2005, 127, 8817;
- 7gJ. Paetzold, J.-E. Bäckvall, J. Am. Chem. Soc. 2005, 127, 17620; for reviews; see:
- 7hO. Pàmies, J.-E. Bäckvall, Chem. Rev. 2003, 103, 3247;
- 7iY. Kim, J. Park, M.-J. Kim, ChemCatChem 2011, 3, 271;
- 7jO. Verho, J.-E. Bäckvall, J. Am. Chem. Soc. 2015, 137, 3996.
- 8A. Berkessel, M. L. Sebastian-Ibarz, T. N. Müller, Angew. Chem. Int. Ed. 2006, 45, 6567; Angew. Chem. 2006, 118, 6717.
- 9
- 9aS. Akai, K. Tanimoto, Y. Kanao, M. Egi, T. Yamamoto, Y. Kita, Angew. Chem. Int. Ed. 2006, 45, 2592; Angew. Chem. 2006, 118, 2654;
- 9bM. Egi, K. Sugiyama, M. Saneto, R. Hanada, K. Kato, S. Akai, Angew. Chem. Int. Ed. 2013, 52, 3654; Angew. Chem. 2013, 125, 3742.
- 10
- 10aS. Wuyts, K. de Temmerman, D. De Vos, P. Jacobs, Chem. Commun. 2003, 1928;
- 10bY. Zhu, K.-L. Fow, G.-K. Chuah, S. Jaenicke, Chem. Eur. J. 2007, 13, 541.
- 11
- 11aT. R. Simmons, G. Berggren, M. Bacchi, M. Fontecave, V. Artero, Coord. Chem. Rev. 2014, 270, 127;
- 11bS. Shima, O. Pilak, S. Vogt, M. Schick, M. S. Stagni, W. Meyer-Klaucke, E. Warkentin, R. K. Thauer, U. Elmer, Science 2008, 321, 572.
- 12For the first synthesis of the iron hydride, see:
- 12aH.-J. Knölker, H. Goesmann, R. Klauss, Angew. Chem. Int. Ed. 1999, 38, 702;
10.1002/(SICI)1521-3773(19990301)38:5<702::AID-ANIE702>3.0.CO;2-W CAS PubMed Web of Science® Google ScholarAngew. Chem. 1999, 111, 727; for catalytic properties, see
- 12bC. P. Casey, H. Guan, J. Am. Chem. Soc. 2007, 129, 5816;
- 12cC. P. Casey, H. Guan, J. Am. Chem. Soc. 2009, 131, 2499; for a recent review on Knölker-type complexes, see:
- 12dA. Quintard, J. Rodriguez, Angew. Chem. Int. Ed. 2014, 53, 4044; Angew. Chem. 2014, 126, 4124; for a review on metal–ligand cooperative catalysis, see:
- 12eR. Khusnutdinova, D. Milstein, Angew. Chem. Int. Ed. 2015, 54, 12236; Angew. Chem. 2015, 127, 12406.
- 13For selected examples on phosphine-free chiral iron complexes, see:
- 13aJ. P. Hopewell, J. E. D. Martins, T. C. Johnson, J. Godfrey, M. Wills, Org. Biomol. Chem. 2012, 10, 134;
- 13bP. Gajewski, M. Renom-Carrasco, S. V. Facchini, L. Pignataro, L. Lefort, J. G. de Vries, R. Ferraccioli, A. Forni, U. Piarulli, C. Gennari, Eur. J. Org. Chem. 2015, 1887; for Knölker-type complexes bearing chiral phosphoramidate, see:
- 13cA. Berkessel, S. Reichau, A. von der Höh, N. Leconte, J.-M. Neudörfl, Organometallics 2011, 30, 3880; for efficient examples of iron-catalyzed asymmetric hydrogenation, see:
- 13dW. Zuo, A. J. Lough, Y. F. Li, R. H. Morris, Science 2013, 342, 1080;
- 13eY. Li, S. Yu, X. Wu, J. Xiao, W. Shen, Z. Dong, J. Gao, J. Am. Chem. Soc. 2014, 136, 4031.
- 14For selected examples of Knölker complexes in asymmetric dual catalysis; see:
- 14aS. Zhou, S. Fleischer, K. Junge, M. Beller, Angew. Chem. Int. Ed. 2011, 50, 5120; Angew. Chem. 2011, 123, 5226;
- 14bA. Quintard, T. Constantieux, J. Rodriguez, Angew. Chem. Int. Ed. 2013, 52, 12883; Angew. Chem. 2013, 125, 13121.
- 15For selected examples of achiral hydrogenation catalyzed by Knölker-type complexes, see:
- 15aS. Fleischer, S. S. Zhou, K. Junge, M. Beller, Angew. Chem. Int. Ed. 2013, 52, 5120; Angew. Chem. 2013, 125, 5224; for transfer hydrogenation, see:
- 15bT. N. Plank, J. L. Drake, D. K. Kim, T. W. Funk, Adv. Synth. Catal. 2012, 354, 597; for oxidation, see:
- 15cM. G. Coleman, A. N. Brown, B. A. Bolton, H. Guan, Adv. Synth. Catal. 2010, 352, 967;
- 15dS. A. Moyer, T. Funk, Tetrahedron Lett. 2010, 51, 5430; for reductive amination, see:
- 15eA. Pagnoux-Ozherelyeva, N. Pannetier, M. D. Mbaye, S. Gaillard, J.-L. Renaud, Angew. Chem. Int. Ed. 2012, 51, 4976; Angew. Chem. 2012, 124, 5060;
- 15fS. Moulin, H. Dentel, A. Pagnoux-Ozherelyeva, S. Gaillard, A. Poater, L. Cavallo, J.-F. Lohier, J.-L. Renaud, Chem. Eur. J. 2013, 19, 17881;
- 15gT.-T. Thai, D. S. Mérel, A. Poater, S. Gaillard, J.-L. Renaud, Chem. Eur. J. 2015, 21, 7066; for amination of alcohols, see:
- 15hT. Yan, B. L. Feringa, K. Barta, Nat. Commun. 2014, 5, 5602;
- 15iA. J. Rawlings, L. J. Diorazio, M. Wills, Org. Lett. 2015, 17, 1086;
- 15jH.-J. Pan, T. W. Ng, Y. Zhao, Chem. Commun. 2015, 51, 11907;
- 15kB. Emayavaramban, M. Roy, B. Sundaraju, Chem. Eur. J. 2016, 22, 3952;
- 15lT. Yan, B. L. Feringa, K. Barta, ACS Catal. 2016, 6, 381; for alkylation of ketones, see:
- 15mS. Elangovan, J.-B. Sortais, M. Beller, C. Darcel, Angew. Chem. Int. Ed. 2015, 54, 14483; Angew. Chem. 2015, 127, 14691.
- 16Cooperative iron and enzyme catalysis for oxidation processes:
- 16aH. Maid, P. Böhm, S. M. Huber, W. Bauer, W. Hummel, N. Jux, H. Gröger, Angew. Chem. Int. Ed. 2011, 50, 2397; Angew. Chem. 2011, 123, 2445; for a recent book chapter on one-pot metal and enzymes catalysis, see:
- 16bH. Gröger, in Cooperative catalysis: Designing Efficient Catalysis for Synthesis (Eds.: ), Wiley-VCH, Weinheim, 2015, pp. 325–349.