Heteroatom Tuning of Bimolecular Criegee Reactions and Its Implications
Graphical Abstract
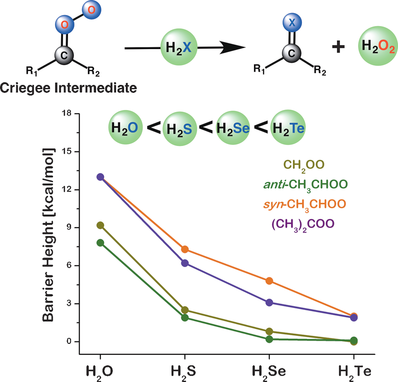
Gas-phase reactions: The calculated barriers for the bimolecular reactions of Criegee intermediates and H2X inversely correlate with the bond strength of the X−H bond of H2X or directly correlate with the first pKa value of H2X. Thus, it is not only the substitutions of Criegee intermediates but also the properties of the heteroatom in H2X that play a crucial role in determining the reactivity.
Abstract
High-level quantum-chemical calculations have been performed to understand the key reactivity determinants of bimolecular reactions of Criegee intermediates and H2X (X=O, S, Se, and Te). Criegee intermediates are implicated as key intermediates in atmospheric, synthetic organic, and enzymatic chemistry. Generally, it is believed that the nature and location of substituents at the carbon of the Criegee intermediate play a key role in determing the reactivity. However, the present work suggests that it is not only the substitution of the Criegee intermediate, but the nature of the heteroatom in H2X that also plays a crucial role in determining the reactivity of the interaction between the Criegee intermediate and H2X. The barriers for the reactions of Criegee intermediates and H2X satisfy an inverse correlation with the bond strength of X−H in H2X, and a direct correlation with the first pKa of H2X. This heteroatom tuning causes a substantial barrier lowering of 8–11 kcal mol−1 in the Criegee reaction barrier in going from H2O to H2Te. An important implication of these results is that the reaction of the Criegee intermediate and H2S could be a source of thioaldehydes, which are important in plantery atmospheres and synthetic organic chemistry. By performing the reaction of Criegee intermediates and H2S under water or acid catalysis, thioladehydes could be detected in a hydrogen-bonded complexed state, which is significantly more stable than their uncomplexed form. As a result, simpler aliphatic thioaldehydes could be selectively synthesized in the laboratory, which, otherwise, has been a significant synthetic challenge because of their ability to oligomerize.