Targeted Covalent Inhibitors for Drug Design
Corresponding Author
Prof. Thomas A. Baillie
Department of Medicinal Chemistry, School of Pharmacy, University of Washington, Box 357610, Seattle, WA, 98195-7610 USA
Search for more papers by this authorCorresponding Author
Prof. Thomas A. Baillie
Department of Medicinal Chemistry, School of Pharmacy, University of Washington, Box 357610, Seattle, WA, 98195-7610 USA
Search for more papers by this authorGraphical Abstract
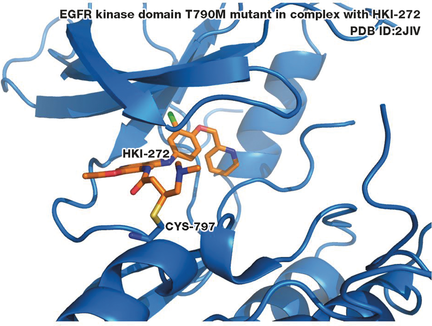
In a bind: Targeted covalent inhibitors (TCIs), such as HKI-272 (shown in the picture in complex with a protein), first form a reversible association and then a covalent bond between an electrophile on the ligand and a nucleophilic center in the protein. This can offer benefits such as high potency and extended duration of action, and new approaches can limit the risk of serious adverse reactions.
Abstract
In contrast to the traditional mechanism of drug action that relies on the reversible, noncovalent interaction of a ligand with its biological target, a targeted covalent inhibitor (TCI) is designed such that the initial, reversible association is followed by the formation of a covalent bond between an electrophile on the ligand and a nucleophilic center in the protein. Although this approach offers a variety of potential benefits (high potency and extended duration of action), concerns over the possible toxicological consequences of protein haptenization have hindered the development of the TCI concept. Recently, approaches to mitigate the risk of serious adverse reactions to this new class of agent have emerged, thus stimulating interest in the field and leading to authorization of the first cadre of TCIs to be marketed. The covalent inhibitor approach is rapidly gaining acceptance as a valuable tool in drug discovery, and is poised to make a major impact on the design of enzyme inhibitors and receptor modulators.
References
- 1J. Singh, R. C. Petter, T. A. Baillie, A. Whitty, Nat. Rev. Drug Discovery 2011, 10, 307–317.
- 2M. H. Potashman, M. E. Duggan, J. Med. Chem. 2009, 52, 1231–1246.
- 3M. C. Noe, A. M. Gilbert, Annu. Rep. Med. Chem. 2012, 47, 413–439.
- 4B. R. Lanning, L. R. Whitby, M. M. Dix, J. Douhan, A. M. Gilbert, E. C. Hett, T. O. Johnson, C. Joslyn, J. C. Kath, S. Niessen, L. R. Roberts, M. E. Schnute, C. Wang, J. J. Hulce, B. Wei, L. O. Whiteley, M. M. Hayward, B. J. Cravatt, Nat. Chem. Biol. 2014, 10, 760–767.
- 5A. J. T. Smith, X. Zhang, A. G. Leach, K. N. Houk, J. Med. Chem. 2009, 52, 225–233.
- 6A. S. Kalgutkar, D. K. Dalvie, Expert Opin. Drug Discovery 2012, 7, 561–581.
- 7D. S. Johnson, E. Weerapana, B. F. Cravatt, Future Med. Chem. 2010, 2, 949–964.
- 8N. Li, H. S. Overkleeft, B. I. Florea, Curr. Opin. Chem. Biol. 2012, 16, 227–233.
- 9R. E. Moellering, B. F. Cravatt, Chem. Biol. 2012, 19, 11–22.
- 10L. E. Sanman, M. Bogyo, Annu. Rev. Biochem. 2014, 83, 249–273.
- 11J. E. Moses, A. D. Moorhouse, Chem. Soc. Rev. 2007, 36, 1249–1262.
- 12M. Schirle, M. Bantscheff, B. Kuster, Chem. Biol. 2012, 19, 72–84.
- 13R. A. Ward, M. J. Anderton, S. Ashton, P. A. Bethel, M. Box, S. Butterworth, N. Colclough, C. G. Chorley, C. Chuaqui, D. A. E. Cross, L. A. Dakin, J. E. Debreczeni, C. Eberlein, M. R. V. Finlay, G. B. Hill, M. Grist, T. C. M. Klinowska, C. Lane, S. Martin, J. P. Orme, P. Smith, F. Wang, M. J. Waring, J. Med. Chem. 2013, 56, 7025–7048.
- 14C.-H. Yun, K. E. Mengwasser, A. V. Toms, M. S. Woo, H. Greulich, K.-K. Wong, M. Meyerson, M. J. Eck, Proc. Natl. Acad. Sci. USA 2008, 105, 2070–2075.
- 15J. Singh, E. M. Dobrusin, D. W. Fry, T. Haske, A. Whitty, D. J. McNamara, J. Med. Chem. 1997, 40, 1130–1135.
- 16D. P. Williams, N. R. Kitteringham, D. J. Naisbitt, M. Pirmohamed, D. A. Smith, B. K. Park, Curr. Drug Metab. 2002, 3, 351–366.
- 17J. C. Erve, Expert Opin. Drug Metab. Toxicol. 2006, 2, 923–946.
- 18F. P. Guengerich, Chem. Res. Toxicol. 2001, 14, 611–650.
- 19S. Zhou, E. Chan, W. Duan, M. Huang, Y. Z. Chen, Drug Metab. Rev. 2005, 37, 41–213.
- 20J. Uetrecht, D. J. Naisbitt, Pharmacol. Rev. 2013, 65, 779–808.
- 21A. J. Wilson, J. K. Kerns, J. F. Callahan, C. J. Moody, J. Med. Chem. 2013, 56, 7463–7476.
- 22D. C. Evans, A. P. Watt, D. A. Nicoll-Griffith, T. A. Baillie, Chem. Res. Toxicol. 2004, 17, 3–16.
- 23T. A. Baillie, Chem. Res. Toxicol. 2006, 19, 889–893.
- 24C. Carmi, A. Lodola, S. Rivara, F. Vacondio, A. Cavazzoni, R. R. Alfieri, P. G. Petronini, M. Mor, Mini-Rev. Med. Chem. 2011, 11, 1019–1030.
- 25J. M. Shin, Y. M. Cho, G. Sachs, J. Am. Chem. Soc. 2004, 126, 7800–7811.
- 26P. M. Dansette, J. Libraire, G. Bertho, D. Mansuy, Chem. Res. Toxicol. 2009, 22, 369–373.
- 27Q. Yongchang, L. Z. Benet, A. L. Burlingame, J. Biol. Chem. 1998, 273, 17940–17953.
- 28D. C. Dahlin, S. D. Nelson, J. Med. Chem. 1982, 25, 885–886.
- 29T. A. Baillie, Drug Metab. Rev. 2015, 47, 4–11.
- 30K.-J. Hoffmann, A. J. Streeter, D. B. Axworthy, T. A. Baillie, Mol. Pharmacol. 1985, 27, 566–573.
- 31T. J. Monks, S. S. Lau, Toxicol. Pathol. 2013, 41, 315–321.
- 32A. F. Kluge, R. C. Petter, Curr. Opin. Chem. Biol. 2010, 14, 421–427.
- 33P.-Y. Yang, K. Liu, M. H. Ngai, M. J. Lear, M. R. Wenk, S. Q. Yao, J. Am. Chem. Soc. 2010, 132, 656–666.
- 34C. Drahl, B. J. Cravatt, E. J. Sorensen, Angew. Chem. Int. Ed. 2005, 44, 5788–5809; Angew. Chem. 2005, 117, 5936–5958.
- 35D. W. Fry, A. J. Bridges, W. A. Denny, A. Doherty, K. D. Greis, J. L. Hicks, K. E. Hook, P. R. Keller, W. R. Leopold, J. A. Loo, D. J. McNamara, J. M. Nelson, V. Sherwood, J. B. Smail, S. Trumpp-Kallmeyer, E. M. Dobrusin, Proc. Natl. Acad. Sci. USA 1998, 95, 12022–12027.
- 36J. Singh, R. C. Petter, A. F. Kluge, Curr. Opin. Chem. Biol. 2010, 14, 475–480.
- 37L. A. Honigberg, A. M. Smith, M. Sirisawad, E. Verner, D. Loury, B. Chang, S. Li, Z. Pan, D. H. Thamm, R. A. Miller, J. J. Buggy, Proc. Natl. Acad. Sci. USA 2010, 107, 13075–13080.
- 38J. B. Smaill, G. W. Rewcastle, J. A. Loo, K. D. Greis, O. H. Chan, E. L. Reyner, E. Lipka, H. D. Showalter, P. W. Vincent, W. L. Elliott, W. A. Denny, J. Med. Chem. 2000, 43, 1380–1397.
- 39W. A. Denny, Pharmacol. Ther. 2002, 93, 253–261.
- 40J. C. Powers, J. L. Asgian, O. D. Ekici, K. E. James, Chem. Rev. 2002, 102, 4639–4750.
- 41M. Hagel, D. Niu, T. St. Martin, M. P. Sheets, L. Qiao, H. Bernard, R. M. Karp, Z. Zhu, M. T. Labenski, P. Chaturvedi, M. Nacht, W. F. Westlin, R. C. Petter, J. Singh, Nat. Chem. Biol. 2011, 7, 22–24.
- 42Q. Zeng, A. G. Nair, S. B. Rosenblum, H.-C. Huang, C. A. Lesburg, Y. Jiang, O. Selyutin, T.-Y. Chan, F. Bennett, K. X. Chen, S. Venkatraman, M. Sannigrahi, F. Velaquez, J. S. Duca, S. Gavalas, Y. Huang, H. Pu, L. Wang, P. Pinto, B. Vibulhan, S. Agrawal, E. Ferrari, C. Jiang, C. Li, D. Hesk, J. Gesell, S. Sorota, N.-Y. Shih, F. G. Njoroge, J. A. Kozlowski, Bioorg. Med. Chem. Lett. 2013, 23, 6585–6587.
- 43X. Koh-Stenta, J. Joy, S. F. Wang, P. Z. Kwek, J. L. K. Wee, K. F. Wan, S. Gayen, A. S. Chen, C. Kang, M. A. Lee, A. Poulsen, S. G. Vasudevan, J. Hill, K. Nacro, Drug Des. Dev. Ther. 2015, 9, 6389–6399.
- 44M. Morgen, C. Jöst, M. Malz, D. Niessing, C. D. Klein, N. Gunkel, A. K. Miller, ACS Chem. Biol. 2016, 11, 1001–1011.
- 45A. M. Omar, M. A. Mahran, M. S. Ghatge, N. Chowdhury, F. H. A. Bamane, M. E. El-Araby, O. Abdulmalik, M. K. Safo, Org. Biomol. Chem. 2015, 13, 6353–6370.
- 46K. Otrubova, M. Brown, M. S. McCormick, G. W. Han, S. T. O'Neal, B. J. Cravatt, R. C. Stevens, A. H. Lichtman, D. L. Boger, J. Am. Chem. Soc. 2013, 135, 6289–6299.
- 47K. Otrubova, B. J. Cravatt, D. L. Boger, J. Med. Chem. 2014, 57, 1079–1089.
- 48M. Cocco, D. Garella, A. Di Stilo, E. Borretto, L. Stevanato, M. Giorgis, E. Marini, R. Fantozzi, G. Miglio, M. Bertinaria, J. Med. Chem. 2014, 57, 10366–10382.
- 49S. Bruno, A. Pinto, G. Paredi, L. Tamborini, C. De Micheli, V. La Pietra, L. Marinelli, E. Novellino, P. Conti, A. Mozzarelli, J. Med. Chem. 2014, 57, 7465–7471.
- 50D. J. Kuhn, Q. Chen, P. M. Voorhees, J. S. Strader, K. D. Shenk, C. M. Sun, S. D. Demo, M. K. Bennett, F. W. Leeuwen, A. A. Chanan-Khan, R. Z. Orlowski, Blood 2007, 110, 3281–3290.
- 51J. Rudolph, D. Stokoe, Angew. Chem. Int. Ed. 2014, 53, 3777–3779; Angew. Chem. 2014, 126, 3851–3853.
- 52K. Sanderson, Nat. Rev. Drug Discovery 2013, 12, 649–651.
- 53F. Liu, X. Zhang, E. Weisberg, S. Chen, W. Hur, H. Wu, Z. Zhao, W. Wang, M. Mao, C. Cai, N. I. Simon, J. Wang, A. T. Look, J. D. Griffin, S. P. Balk, Q. Liu, N. S. Gray, ACS Chem. Biol. 2013, 8, 1423–1428.
- 54W. Zhou, W. Hur, U. McDermott, A. Dutt, W. Xian, S. B. Ficarro, J. Zhang, S. V. Sharma, J. Brugge, M. Meyerson, J. Settleman, N. S. Gray, Chem. Biol. 2010, 17, 285–295.
- 55D. L. Perez, V. Palomo, C. Perez, C. Gil, P. D. Dans, F. J. Luque, S. Conde, A. Martinez, J. Med. Chem. 2011, 54, 4042–4056.
- 56C. W. Zapf, B. S. Gerstenberger, L. Xing, D. C. Limburg, D. R. Anderson, N. Caspers, S. Han, A. Aulabaugh, R. Kurumbail, S. Shakya, X. Li, V. Spaulding, R. M. Czerwinski, N. Seth, Q. G. Medley, J. Med. Chem. 2012, 55, 10047–10063.
- 57T. Zhang, F. Inesta-Vaquera, M. Niepel, J. Zhang, S. B. Ficarro, T. Machleidt, T. Xie, J. A. Marto, N. Kim, T. Sim, J. D. Laughlin, H. Park, P. V. LoGrasso, M. Patricelli, T. K. Nomanbhoy, P. K. Sorger, D. R. Alessi, N. S. Gray, Chem. Biol. 2012, 19, 140–154.
- 58E. Leproult, S. Barluenga, D. Moras, J. M. Wurtz, N. Wissinger, J. Med. Chem. 2011, 54, 1347–1355.
- 59J. C. Henise, J. Taunton, J. Med. Chem. 2011, 54, 4133–4146.
- 60M. Nacht, L. Qiao, M. P. Sheets, T. St. Martin, M. Labenski, H. Mazdiyasni, R. Karp, Z. Zhu, P. Charurvedi, D. Bhavsar, D. Niu, W. Westlin, R. C. Petter, A. P. Medikonda, J. Singh, J. Med. Chem. 2013, 56, 712–721.
- 61M. S. Cohen, C. Zhang, K. M. Shokat, J. Taunton, Science 2005, 308, 1318–1321.
- 62A. K. Devkota, R. Edupuganti, C. Yan, Y. Shi, J. Jose, Q. Wang, T. S. Kaoud, E. J. Cho, P. Ren, K. N. Dalby, ChemBioChem 2014, 15, 2435–2442.
- 63F. E. Kwarcinski, C. C. Fox, M. E. Steffey, M. B. Soellner, ACS Chem. Biol. 2012, 7, 1910–1917.
- 64N. N. Gushwa, S. Kang, J. Chen, J. Taunton, J. Am. Chem. Soc. 2012, 134, 20214–20217.
- 65L. Xing, A. Huang, Future Med. Chem. 2014, 6, 675–695.
- 66M. F. Moghaddam, Y. Tang, Z. O'Brien, S. J. Richardson, M. Bacolod, P. Chaturvedi, J. Apuy, A. Kulkarni, Drug Metab. Lett. 2014, 8, 19–30.
- 67T. Barf, A. Kaptein, J. Med. Chem. 2012, 55, 6243–6262.
- 68Q. Liu, Y. Sabnis, Z. Zhao, T. Zhang, S. J. Buhrlage, L. H. Jones, N. Gray, Chem. Biol. 2013, 20, 146–159.
- 69L. Garuti, M. Roberti, G. Bottegoni, Curr. Med. Chem. 2011, 18, 2981–2994.
- 70A. S. Kalgutkar, D. Dalvie, R. S. Obach, D. A. Smith in Reactive Drug Metabolites, Methods and Principles in Medicinal Chemistry, Vol. 55 (Eds.: ), Wiley-VCH, Weinheim, 2012, pp. 313–334.
- 71L. Kubiczkova, L. Pour, L. Sedlankova, R. Hajek, S. Sevcikova, J. Cell. Mol. Med. 2014, 18, 947–961.
- 72D. A. Bachovchin, B. F. Cravatt, Nat. Rev. Drug Discovery 2012, 11, 52–68.
- 73J. J. M. Wiener, S. Sun, R. L. Thurmond, Curr. Top. Med. Chem. 2010, 10, 717–732.
- 74A. Smelcerovic, F. Miljkovic, J. Lazarevic, A. Djorjevic, G. Kocic, M. Anderluh, Top. Med. Chem. 2015, 15, 2342–2372.
- 75P. A. Schwartz, P. Kuzmic, J. Solowiej, S. Bergqvist, B. Bolanos, C. Almaden, A. Nagata, K. Ryan, J. Feng, D. Dalvie, J. C. Kath, M. Xu, R. Wani, B. W. Murray, Proc. Natl. Acad. Sci. USA 2014, 111, 173–178.
- 76S. Parmar, K. Patel, J. Pinilla-Ibarz, Pharm. Ther. 2014, 39, 483–487.
- 77I. M. Serafimova, M. A. Pufall, S. Krishnan, K. Duda, M. S. Cohen, R. L. Maglathlin, J. M. McGarland, R. M. Miller, M. Frödin, J. Taunton, Nat. Chem. Biol. 2012, 8, 471–476.
- 78R. M. Miller, V. O. Paavilainen, S. Krishnan, I. M. Serafimova, J. Taunton, J. Am. Chem. Soc. 2013, 135, 5298–5301.
- 79S. Krishnan, R. M. Miller, B. Tian, R. D. Mullins, M. P. Jacobson, J. Taunton, J. Am. Chem. Soc. 2014, 136, 12624–12630.
- 80R. M. Miller, J. Taunton, Methods Enzymol. 2014, 548, 93–116.
- 81G. Xia, W. Chen, J. Zhang, J. Shao, Y. Zhang, W. Huang, L. Zhang, W. Qi, X. Sun, B. Li, Z. Xiang, C. Ma, J. Xu, H. Deng, Y. Li, P. Li, H. Miao, J. Han, Y. Liu, J. Shen, Y. Yu, J. Med. Chem. 2014, 57, 9889–9900.
- 82D. Basu, A. Richters, D. Rauh, Bioorg. Med. Chem. 2015, 23, 2767–2780.
- 83R. Mah, J. R. Thomas, C. M. Shafer, Bioorg. Med. Chem. Lett. 2014, 24, 33–39.
- 84M. H. Johansson, Mini-Rev. Med. Chem. 2012, 12, 1330–1344.
- 85Y. Shibata, M. Chiba, Drug Metab. Dipos. 2015, 43, 375–384.
- 86P. Stopfer, K. Marzin, H. Narjes, D. Gansser, M. Shahidi, M. Uttereuther-Fischer, T. Ebner, Cancer Chemother. Pharmacol. 2012, 69, 1051–1061.
- 87R. Abbas, B. A. Hug, C. Leister, J. Burns, D. Sonnichsen, Br. J. Clin. Pharmacol. 2011, 71, 522–527.
- 88E. Scheers, L. Leclercq, J. de Jong, N. Bode, M. Bockx, A. Laenen, F. Cuyckens, D. Skee, J. Murphy, J. Sukbuntherng, G. Mannens, Drug Metab. Dispos. 2014, 43, 289–297.
- 89A. Chandrasekaran, L. Shen, S. Lockhead, A. Oganesian, J. Wang, J. Scatina, Drug Metab. Lett. 2010, 4, 220–227.
- 90J. Wang, X. Li-Chan, J. Atherton, L. Deng, R. Espina, L. Yu, P. Horwatt, S. Ross, S. Lockhead, S. Ahmad, A. Chandrasakeran, A. Oganesian, J. Scatina, A. Mutlib, R. Talaat, Drug Metab. Dispos. 2010, 38, 1083–1093.
- 91D. Lin, S. Saleh, D. C. Liebler, Chem. Res. Toxicol. 2008, 21, 2361–2369.
- 92L. H. M. Vroomen, M. C. Berghmans, J. P. Groten, J. H. Koeman, P. J. van Bladeren, Toxicol. Appl. Pharmacol. 1988, 95, 53–60.
- 93T. A. Baillie, J. G. Slatter, Acc. Chem. Res. 1991, 24, 264–270.
- 94U. P. Dahal, R. S. Obach, A. M. Gilbert, Chem. Res. Toxicol. 2013, 26, 1739–1745.
- 95M. E. Flanagan, J. A. Abramite, D. P. Anderson, A. Aulbaugh, U. P. Dahal, A. M. Gilbert, C. Li, J. Montgomery, S. R. Oppenheimer, T. Ryder, B. P. Schuff, D. P. Uccello, G. S. Walker, Y. Wu, M. F. Brown, J. M. Chen, M. W. Hayward, M. C. Noe, R. S. Obach, L. Philippe, V. Shanmugasundaram, M. J. Shapiro, J. Starr, J. Stroh, Y. Che, J. Med. Chem. 2014, 57, 10072–10079.
- 96R. M. Oballa, J.-F. Truchon, C. I. Bayly, N. Chauret, S. Day, S. Crane, C. Berthelette, Bioorg. Med. Chem. Lett. 2007, 17, 998–1002.
- 97A. Böhme, D. Thaens, A. Paschke, G. Schüürmann, Chem. Res. Toxicol. 2009, 22, 742–750.
- 98P. A. MacFaul, A. D. Morely, J. J. Crawford, Bioorg. Med. Chem. Lett. 2009, 19, 1136–1138.
- 99T. E. Ballard, U. P. Dahal, A. J. Bessire, R. P. Schneider, K. F. Geoghegan, A. D. N. Vaz, Rapid Commun. Mass Spectrom. 2015, 29, 2175–2183.
- 100M. Gersch, M. W. Hackl, C. Dubiella, A. Dobrinevski, M. Groll, S. A. Sieber, Chem. Biol. 2015, 22, 404–411.
- 101I. D. G. Campuzano, T. S. Miguel, T. Rowe, D. Onea, V. J. Cee, T. Arvedson, J. D. McCarter, J. Biomol. Screening 2015, 1–9.
- 102Y. Yang, Y.-Z. Shu, W. G. Humphreys, Chem. Res. Toxicol. 2016, 29, 109–116.
- 103R. Guerciolini, Int. J. Obes. 1997, 21, S 12–S23.
- 104S. Kumar, K. Kassahun, R. A. Tschirret-Guth, K. Mitra, T. A. Baillie, Curr. Opin. Drug Discovery Dev. 2008, 11, 43–52.
- 105T. J. Strelevitz, X. Yang, L. Wood, J.-C. Marshall, M. Zientek, L. Tremaine, L. Leung, Drug Metab. Rev. 2015, 47(S1), 59.
- 106J. D. Hughes, J. Blagg, D. A. Price, S. Bailey, G. A. DeCrescenzo, R. V. Devraj, E. Ellsworth, Y. M. Fobian, M. E. Gibbs, R. W. Gilles, N. Greene, E. Huang, T. Krieger-Burke, J. Loesel, T. Wager, L. Whiteley, Y. Zhang, Bioorg. Med. Chem. Lett. 2008, 18, 4872–4875.
- 107The Wall Street Journal, December 2, 2015.
- 108D. Butler, E. Callaway, Nature 2016, 529, 263–264.
- 109C. Lammert, S. Einarsson, C. Saha, A. Niklasson, E. Bjornsson, N. Chalasani, Hepatology 2008, 47, 2003–2009.
- 110M. Chen, J. Borlak, W. Tong, Hepatology 2013, 58, 388–396.
- 111A. F. Stepan, D. P. Walker, J. Bauman, D. A. Price, T. A. Baillie, A. S. Kalgutkar, M. D. Aleo, Chem. Res. Toxicol. 2011, 24, 1345–1410.
- 112V. J. Cee, L. P. Volak, Y. Chen, M. D. Bartberger, C. Tegley, T. Arvedson, J. McCarter, A. S. Tasker, C. Fotsch, J. Med. Chem. 2015, 58, 9171–9178.
- 113P. M. S. D. Cal, J. B. Vicente, E. Pires, A. V. Coelho, L. F. Veiros, C. Cordeiro, P. M. P. Gois, J. Am. Chem. Soc. 2012, 134, 10299–10305.
- 114C. Jöst, C. Nitsche, T. Scholz, L. Roux, C. D. Klein, J. Med. Chem. 2014, 57, 7590–7599.
- 115A. Berteotti, F. Vacondio, A. Lodola, M. Bassi, C. Silva, M. Mor, A. Cavalli, ACS Med. Chem. Lett. 2014, 5, 501–505.
- 116J. Schröder, A. Klinger, F. Oellien, R. J. Marhöfer, M. Duszenko, P. M. Selzer, J. Med. Chem. 2013, 56, 1478–1490.
- 117S. G. Kathman, Z. Xu, A. V. Statsyuk, J. Med. Chem. 2014, 57, 4969–4974.
- 118N. Godin-Heymann, L. Ulkas, W. B. Brannigan, U. McDermott, J. Lamb, S. Maheswaran, J. Settleman, D. A. Haber, Mol. Cancer Ther. 2008, 7, 874–879.
- 119D. Ercan, H. G. Choi, C.-H. Yun, M. Capalletti, T. Xie, M. J. Eck, N. S. Gray, P. A. Jänne, Clin. Cancer Res. 2015, 21, 3913–3923.
- 120J. C. Way, Curr. Opin. Chem. Biol. 2000, 4, 40–46.
- 121H. M. Dickson, A. Wilbur, A. A. Reinke, M. A. Young, A. B. Vojtek, BMC Neurosci. 2015, 16, 34.
- 122R. Nussinov, C.-J. Tsai, Annu. Rev. Pharmacol. Toxicol. 2015, 55, 249–267.