Iron-Carbonyl-Catalyzed Redox-Neutral [4+2] Annulation of N−H Imines and Internal Alkynes by C−H Bond Activation
Teng Jia
Beijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
Search for more papers by this authorChongyang Zhao
BNLMS, CAS Key Laboratory of Photochemistry, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
Search for more papers by this authorDr. Ruoyu He
Beijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hui Chen
BNLMS, CAS Key Laboratory of Photochemistry, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Congyang Wang
Beijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
Search for more papers by this authorTeng Jia
Beijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
Search for more papers by this authorChongyang Zhao
BNLMS, CAS Key Laboratory of Photochemistry, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
Search for more papers by this authorDr. Ruoyu He
Beijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hui Chen
BNLMS, CAS Key Laboratory of Photochemistry, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Congyang Wang
Beijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
Search for more papers by this authorGraphical Abstract
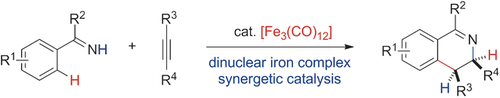
Double dose of iron: The titled redox-neutral [4+2] annulations to furnish cis-3,4-dihydroisoquinolines were achieved by using iron catalysis. Mechanistic studies show the synergy of dinuclear iron in the C−H bond activation and turnover-limiting hydrogen-transfer steps. The reaction demonstrates excellent atom economy and exclusive cis stereoselectivity.
Abstract
Stoichiometric C−H bond activation of arenes mediated by iron carbonyls was reported by Pauson as early as in 1965, yet the catalytic C−H transformations have not been developed. Herein, an iron-catalyzed annulation of N−H imines and internal alkynes to furnish cis-3,4-dihydroisoquinolines is described, and represents the first iron-carbonyl-catalyzed C−H activation reaction of arenes. Remarkablely, this is also the first redox-neutral [4+2] annulation of imines and alkynes proceeding by C−H activation. The reaction also features only cis stereoselectivity and excellent atom economy as neither base, nor external ligand, nor additive is required. Experimental and theoretical studies reveal an oxidative addition mechanism for C−H bond activation to afford a dinuclear ferracycle and a synergetic diiron-promoted H-transfer to the alkyne as the turnover-determining step.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201600365-sup-0001-misc_information.pdf4.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. Q. Yu, Z.-J. Shi, C−H Activation, Springer, Berlin, 2010.
- 2For reviews:
- 2aA. A. Kulkarni, O. Daugulis, Synthesis 2009, 4087;
- 2bC.-J. Li, Acc. Chem. Res. 2009, 42, 335;
- 2cE. Nakamura, N. Yoshikai, J. Org. Chem. 2010, 75, 6061;
- 2dC.-L. Sun, B.-J. Li, Z.-J. Shi, Chem. Rev. 2011, 111, 1293;
- 2eC. Zhang, C. Tang, N. Jiao, Chem. Soc. Rev. 2012, 41, 3464;
- 2fM. C. White, Science 2012, 335, 807;
- 2gC. Wang, Synlett 2013, 1606;
- 2hF. Jia, Z. Li, Org. Chem. Front. 2014, 1, 194;
- 2iK. Gao, N. Yoshikai, Acc. Chem. Res. 2014, 47, 1208;
- 2jL. Ackermann, J. Org. Chem. 2014, 79, 8948.
- 3I. Bauer, H.-J. Knölker, Chem. Rev. 2015, 115, 3170.
- 4For representative examples:
- 4aJ. Norinder, A. Matsumoto, N. Yoshikai, E. Nakamura, J. Am. Chem. Soc. 2008, 130, 5858;
- 4bN. Yoshikai, A. Matsumoto, J. Norinder, E. Nakamura, Angew. Chem. Int. Ed. 2009, 48, 2925; Angew. Chem. 2009, 121, 2969;
- 4cL. Ilies, S. Asako, E. Nakamura, J. Am. Chem. Soc. 2011, 133, 7672;
- 4dR. Shang, L. Ilies, A. Matsumoto, E. Nakamura, J. Am. Chem. Soc. 2013, 135, 6030;
- 4eS. Asako, L. Ilies, E. Nakamura, J. Am. Chem. Soc. 2013, 135, 17755;
- 4fQ. Gu, H. H. Al Mamari, K. Graczyk, E. Diers, L. Ackermann, Angew. Chem. Int. Ed. 2014, 53, 3868; Angew. Chem. 2014, 126, 3949;
- 4gB. M. Monks, E. R. Fruchey, S. P. Cook, Angew. Chem. Int. Ed. 2014, 53, 11065; Angew. Chem. 2014, 126, 11245;
- 4hT. Matsubara, S. Asako, L. Ilies, E. Nakamura, J. Am. Chem. Soc. 2014, 136, 646;
- 4iL. Ilies, T. Matsubara, S. Ichikawa, S. Asako, E. Nakamura, J. Am. Chem. Soc. 2014, 136, 13126;
- 4jE. R. Fruchey, B. M. Monks, S. P. Cook, J. Am. Chem. Soc. 2014, 136, 13130;
- 4kJ. J. Sirois, R. Davis, B. DeBoef, Org. Lett. 2014, 16, 868;
- 4lR. Shang, L. Ilies, E. Nakamura, J. Am. Chem. Soc. 2015, 137, 7660;
- 4mK. Graczyk, T. Haven, L. Ackermann, Chem. Eur. J. 2015, 21, 8812;
- 4nM. Y. Wong, T. Yamakawa, N. Yoshikai, Org. Lett. 2015, 17, 442;
- 4oG. Cera, T. Haven, L. Ackermann, Angew. Chem. Int. Ed. 2016, 55, 1484; Angew. Chem. 2016, 128, 1506.
- 5For selected examples:
- 5aM. M. Bagga, P. L. Pauson, F. J. Preston, R. I. Reed, J. Chem. Soc. Chem. Commun. 1965, 543;
- 5bP. E. Baikie, O. S. Mills, Chem. Commun. 1966, 707;
- 5cM. M. Bagga, W. T. Flannigan, G. R. Knox, P. L. Pauson, F. J. Preston, R. I. Reed, J. Chem. Soc. C 1968, 36;
- 5dW. T. Flannigan, G. R. Knox, P. L. Pauson, J. Chem. Soc. C 1969, 2077;
- 5eN. S. Nametkin, V. D. Tyurin, A. I. Nekhaev, Yu. P. Sobolev, M. G. Kondrat'eva, A. S. Batsanov, Yu. T. Struchkov, J. Organomet. Chem. 1983, 243, 323;
- 5fN. S. Nametkin, V. D. Tyurin, V. V. Trusov, A. M. Krapivin, J. Organomet. Chem. 1983, 254, 243;
- 5gW. Imhof, J. Organomet. Chem. 1997, 533, 31;
- 5hD.-L. Wang, W.-S. Hwang, L.-C. Liang, L.-I. Wang, L. Lee, M. Y. Chiang, Organometallics 1997, 16, 3109;
- 5iW. Imhof, Organometallics 1999, 18, 4845.
- 6For selected examples:
- 6aY. Kuninobu, A. Kawata, K. Takai, J. Am. Chem. Soc. 2005, 127, 13498;
- 6bZ.-M. Sun, S.-P. Chen, P. Zhao, Chem. Eur. J. 2010, 16, 2619;
- 6cD. N. Tran, N. Cramer, Angew. Chem. Int. Ed. 2011, 50, 11098; Angew. Chem. 2011, 123, 11294;
- 6dP. Zhao, F. Wang, K. Han, X. Li, Org. Lett. 2012, 14, 5506;
- 6eJ. Zhang, A. Ugrinov, P. Zhao, Angew. Chem. Int. Ed. 2013, 52, 6681; Angew. Chem. 2013, 125, 6813;
- 6fL. Dong, C.-H. Qu, J.-R. Huang, W. Zhang, Q.-R. Zhang, J.-G. Deng, Chem. Eur. J. 2013, 19, 16537;
- 6gM. Nagamoto, T. Nishimura, Chem. Commun. 2014, 50, 6274;
- 6hX. Jin, X. Yang, Y. Yang, C. Wang, Org. Chem. Front. 2016, 3, 268.
- 7For a review:
- 7aR. He, Z.-T. Huang, Q.-Y. Zheng, C. Wang, Tetrahedron Lett. 2014, 55, 5705; For selected examples:
- 7bN. Guimond, K. Fagnou, J. Am. Chem. Soc. 2009, 131, 12050;
- 7cT. Fukutani, N. Umeda, K. Hirano, T. Satoh, M. Miura, Chem. Commun. 2009, 5141;
- 7dX. Wei, M. Zhao, Z. Du, X. Li, Org. Lett. 2011, 13, 4636;
- 7eT. K. Hyster, T. Rovis, Chem. Commun. 2011, 47, 11846;
- 7fC. Kornhaaß, J. Li, L. Ackermann, J. Org. Chem. 2012, 77, 9190;
- 7gH. Wang, C. Grohmann, C. Nimphius, F. Glorius, J. Am. Chem. Soc. 2012, 134, 19592;
- 7hL. Qiu, D. Huang, G. Xu, Z. Dai, J. Sun, Org. Lett. 2015, 17, 1810.
- 8R. He, Z.-T. Huang, Q.-Y. Zheng, C. Wang, Angew. Chem. Int. Ed. 2014, 53, 4950; Angew. Chem. 2014, 126, 5050.
- 9For more details, see the Supporting Information.
- 10
- 10aR. P. Dodge, V. Schomaker, J. Organomet. Chem. 1965, 3, 274;
- 10bJ. F. Blount, L. F. Dahl, C. Hoogzand, W. Hübel, J. Am. Chem. Soc. 1966, 88, 292;
- 10cD. Osella, R. Gobetto, P. Montangero, Organometallics 1986, 5, 1247.
- 11R. S. Manan, P. Kilaru, P. Zhao, J. Am. Chem. Soc. 2015, 137, 6136.
- 12E. M. Simmons, J. F. Hartwig, Angew. Chem. Int. Ed. 2012, 51, 3066; Angew. Chem. 2012, 124, 3120.
- 13CCDC 1445220 contains the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.