Increasing Electron-Transfer Rates with Increasing Donor–Acceptor Distance
Graphical Abstract
Abstract
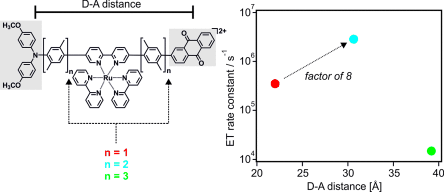
Electron transfer can readily occur over long (≥15 Å) distances. Usually reaction rates decrease with increasing distance between donors and acceptors, but theory predicts a regime in which electron-transfer rates increase with increasing donor–acceptor separation. This counter-intuitive behavior can result from the interplay of reorganization energy and electronic coupling, but until now experimental studies have failed to provide unambiguous evidence for this effect. We report here on a homologous series of rigid rodlike donor-bridge-acceptor compounds in which the electron-transfer rate increases by a factor of 8 when the donor–acceptor distance is extended from 22.0 to 30.6 Å, and then it decreases by a factor of 188 when the distance is increased further to 39.2 Å. This effect has important implications for solar energy conversion.