O2 Activation and Double CH Oxidation by a Mononuclear Manganese(II) Complex
Graphical Abstract
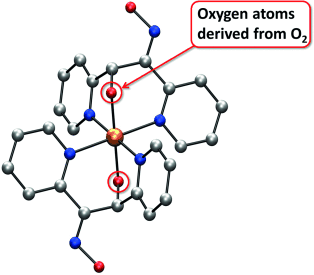
A mononuclear manganese(II) complex of a new oxime-dipyridyl ligand activates O2. Catalytic methylene CH oxidation of the ligand then yields an alkoxide and finally a ketone in a stepwise process. Bis(manganese) complexes of the ligand in its various stages of oxidation were structurally characterized.
Abstract
A MnII complex, [Mn(dpeo)2]2+ (dpeo=1,2-di(pyridin-2-yl)ethanone oxime), activates O2, with ensuing stepwise oxidation of the methylene group in the ligands providing an alkoxide and ultimately a ketone group. X-ray crystal-structure analysis of an intermediate homoleptic alkoxide MnIII complex shows tridentate binding of the ligand via the two pyridyl groups and the newly installed alkoxide moiety, with the oxime group no longer coordinated. The structure of a MnII complex of the final ketone ligand, cis-[MnBr2(hidpe)2] (hidpe=2-(hydroxyimino)-1,2-di(pyridine-2-yl)ethanone) shows that bidentate oxime/pyridine coordination has been resumed. H218O and 18O2 labeling experiments suggest that the inserted O atoms originate from two different O2 molecules. The progress of the oxygenation was monitored through changes in the resonance-enhanced Raman bands of the oxime unit.