Thermal Dehydrogenation of Base-Stabilized B2H5+ Complexes and Its Role in CH Borylation
Corresponding Author
Dr. Aleksandrs Prokofjevs
Department of Chemistry, University of Michigan, 930 N. University Ave, Ann Arbor, MI 48109 (USA)
Current address: Department of Chemistry, Northwestern University, 2145 Sheridan Rd, Evanston, IL 60208 (USA)
Department of Chemistry, University of Michigan, 930 N. University Ave, Ann Arbor, MI 48109 (USA)Search for more papers by this authorCorresponding Author
Dr. Aleksandrs Prokofjevs
Department of Chemistry, University of Michigan, 930 N. University Ave, Ann Arbor, MI 48109 (USA)
Current address: Department of Chemistry, Northwestern University, 2145 Sheridan Rd, Evanston, IL 60208 (USA)
Department of Chemistry, University of Michigan, 930 N. University Ave, Ann Arbor, MI 48109 (USA)Search for more papers by this authorGraphical Abstract
Abstract
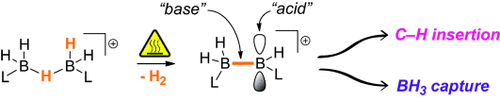
Thermally induced dehydrogenation of the H-bridged cation L2B2H5+ (L=Lewis base) is proposed to be the key step in the intramolecular CH borylation of tertiary amine boranes activated with catalytic amounts of strong “hydridophiles”. Loss of H2 from L2B2H5+ generates the highly reactive cation L2B2H3+, which in its sp2-sp3 diborane(4) form then undergoes either an intramolecular CH insertion with BB bond cleavage, or captures BH3 to produce L2B3H6+. The effect of the counterion stability on the outcome of the reaction is illustrated by formation of LBH2C6F5 complexes through disproportionation of L2B2H5+ HB(C6F5)3−.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201507647_sm_miscellaneous_information.pdf4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aF. H. Stephens, R. T. Baker, M. H. Matus, D. J. Grant, D. A. Dixon, Angew. Chem. Int. Ed. 2007, 46, 746–749; Angew. Chem. 2007, 119, 760–763;
- 1bA. J. M. Miller, J. E. Bercaw, Chem. Commun. 2010, 46, 1709–1711;
- 1cO. J. Metters, A. M. Chapman, A. P. M. Robertson, C. H. Woodall, P. J. Gates, D. F. Wass, I. Manners, Chem. Commun. 2014, 50, 12146–12149;
- 1dFor a recent review of amine borane dehydrogenations, see: N. E. Stubbs, A. P. M. Robertson, E. M. Leitao, I. Manners, J. Organomet. Chem. 2013, 730, 84–89.
- 2Electrophilic CH borylations initiated by hydride abstraction from L-BH3:
- 2aT. S. De Vries, A. Prokofjevs, J. N. Harvey, E. Vedejs, J. Am. Chem. Soc. 2009, 131, 14679–14687;
- 2bA. Prokofjevs, E. Vedejs, J. Am. Chem. Soc. 2011, 133, 20056–20059;
- 2cA. Prokofjevs, J. Jermaks, A. Borovika, J. W. Kampf, E. Vedejs, Organometallics 2013, 32, 6701–6711;
- 2dC. Cazorla, T. S. De Vries, E. Vedejs, Org. Lett. 2013, 15, 984–987;
- 2eT. Stahl, K. Müther, Y. Ohki, K. Tatsumi, M. Oestreich, J. Am. Chem. Soc. 2013, 135, 10978–10981;
- 2fJ. M. Farrell, D. W. Stephan, Angew. Chem. Int. Ed. 2015, 54, 5214–5217; Angew. Chem. 2015, 127, 5303–5306.
- 3Other representative electrophilic CH borylations:
- 3aA. Del Grosso, J. Ayuso Carrillo, M. J. Ingleson, Chem. Commun. 2015, 51, 2878–2881;
- 3bV. Bagutski, A. Del Grosso, J. A. Carrillo, I. A. Cade, M. D. Helm, J. R. Lawson, P. J. Singleton, S. A. Solomon, T. Marcelli, M. J. Ingleson, J. Am. Chem. Soc. 2013, 135, 474–487;
- 3cS. A. Solomon, A. Del Grosso, E. R. Clark, V. Bagutski, J. J. W. McDouall, M. J. Ingleson, Organometallics 2012, 31, 1908–1916;
- 3dA. Prokofjevs, J. W. Kampf, E. Vedejs, Angew. Chem. Int. Ed. 2011, 50, 2098–2101; Angew. Chem. 2011, 123, 2146–2149;
- 3eA. Del Grosso, M. D. Helm, S. A. Solomon, D. Caras-Quintero, M. J. Ingleson, Chem. Commun. 2011, 47, 12459–12461;
- 3fA. Del Grosso, P. J. Singleton, C. A. Muryn, M. J. Ingleson, Angew. Chem. Int. Ed. 2011, 50, 2102–2106; Angew. Chem. 2011, 123, 2150–2154;
- 3gN. Ishida, T. Moriya, T. Goya, M. Murakami, J. Org. Chem. 2010, 75, 8709–8712;
- 3hA. Del Grosso, R. G. Pritchard, C. A. Muryn, M. J. Ingleson, Organometallics 2010, 29, 241–249. Also see Ref. [6a] and references therein.
- 4Transition metal catalyzed BB dehydrocoupling:
- 4aO. Ciobanu, E. Kaifer, M. Enders, H.-J. Himmel, Angew. Chem. Int. Ed. 2009, 48, 5538–5541; Angew. Chem. 2009, 121, 5646–5649;
- 4bH. C. Johnson, C. L. McMullin, S. D. Pike, S. A. Macgregor, A. S. Weller, Angew. Chem. Int. Ed. 2013, 52, 9776–9780; Angew. Chem. 2013, 125, 9958–9962;
- 4cA. Wagner, S. Litters, J. Elias, E. Kaifer, H.-J. Himmel, Chem. Eur. J. 2014, 20, 12514–12527;
- 4dS. Muhammad, S. Moncho, E. N. Brothers, A. A. Bengali, Chem. Commun. 2014, 50, 5874–5877.
- 5A. Staubitz, A. P. M. Robertson, I. Manners, Chem. Rev. 2010, 110, 4079–4124.
- 6
- 6aM. J. Ingleson, Top. Organomet. Chem. 2015, 49, 39–71;
- 6bT. S. De Vries, A. Prokofjevs, E. Vedejs, Chem. Rev. 2012, 112, 4246–4282;
- 6cW. E. Piers, S. C. Bourke, K. D. Conroy, Angew. Chem. Int. Ed. 2005, 44, 5016–5036; Angew. Chem. 2005, 117, 5142–5163;
- 6dP. Kölle, H. Nöth, Chem. Rev. 1985, 85, 399–418.
- 7
- 7aM. A. Dureen, A. Lough, T. M. Gilbert, D. W. Stephan, Chem. Commun. 2008, 4303–4305;
- 7bD. McArthur, C. P. Butts, D. M. Lindsay, Chem. Commun. 2011, 47, 6650–6652;
- 7cJ. M. Farrell, J. A. Hatnean, D. W. Stephan, J. Am. Chem. Soc. 2012, 134, 15728–15731;
- 7dA. Solovyev, S. J. Geib, E. Lacôte, D. P. Curran, Organometallics 2012, 31, 54–56;
- 7eP. Eisenberger, B. P. Bestvater, E. C. Keske, C. M. Crudden, Angew. Chem. Int. Ed. 2015, 54, 2467–2471; Angew. Chem. 2015, 127, 2497–2501.
- 8A. Prokofjevs, J. W. Kampf, A. Solovyev, D. P. Curran, E. Vedejs, J. Am. Chem. Soc. 2013, 135, 15686–15689.
- 9
- 9aM. Kameda, G. Kodama, Inorg. Chem. 1997, 36, 4369–4371;
- 9bT. S. De Vries, E. Vedejs, Organometallics 2007, 26, 3079–3081;
- 9cB. Inés, M. Patil, J. Carreras, R. Goddard, W. Thiel, M. Alcarazo, Angew. Chem. Int. Ed. 2011, 50, 8400–8403; Angew. Chem. 2011, 123, 8550–8553;
- 9dA. Prokofjevs, A. Boussonnière, L. Li, H. Bonin, E. Lacôte, D. P. Curran, E. Vedejs, J. Am. Chem. Soc. 2012, 134, 12281–12288.
- 10
- 10aP. Bissinger, H. Braunschweig, A. Damme, R. D. Dewhurst, T. Kupfer, K. Radacki, K. Wagner, J. Am. Chem. Soc. 2011, 133, 19044–19047;
- 10bD. P. Curran, A. Boussonnière, S. J. Geib, E. Lacôte, Angew. Chem. Int. Ed. 2012, 51, 1602–1605; Angew. Chem. 2012, 124, 1634–1637;
- 10cR. S. Ghadwal, C. J. Schürmann, F. Engelhardt, C. Steinmetzger, Eur. J. Inorg. Chem. 2014, 4921–4926.
- 11Substantial BB bond polarization is consistent with previous theoretical investigations on sp2sp3 diboranes(4) used as nucleophilic boron sources:
- 11aN. Miralles, J. Cid, A. B. Cuenca, J. J. Carbó, E. Fernández, Chem. Commun. 2015, 51, 1693–1696;
- 11bA. Bonet, C. Pubill-Ulldemolins, C. Bo, H. Gulyás, E. Fernández, Angew. Chem. Int. Ed. 2011, 50, 7158–7161; Angew. Chem. 2011, 123, 7296–7299;
- 11cC. Pubill-Ulldemolins, A. Bonet, C. Bo, H. Gulyás, E. Fernández, Chem. Eur. J. 2012, 18, 1121–1126.
- 12Facile hydride exchange in L2B2H5+ was previously confirmed by NMR studies. See Refs. [2a,b] and [9b].
- 13
- 13aR. D. Dewhurst, E. C. Neeve, H. Braunschweig, T. B. Marder, Chem. Commun. 2015, 51, 9594–9607, and references therein;
- 13bH. Asakawa, K.-H. Lee, Z. Lin, M. Yamashita, Nat. Commun. 2014, 5, 4245;
- 13cS. Pietsch, E. C. Neeve, D. C. Apperley, R. Bertermann, F. Mo, D. Qiu, M. S. Cheung, L. Dang, J. Wang, U. Radius, Z. Lin, C. Kleeberg, T. B. Marder, Chem. Eur. J. 2015, 21, 7082–7098;
- 13dsp2-sp2 diborane(4) cations: H. Braunschweig, A. Damme, R. D. Dewhurst, T. Kramer, T. Kupfer, K. Radacki, E. Siedler, A. Trumpp, K. Wagner, C. Werner, J. Am. Chem. Soc. 2013, 135, 8702–8707;
- 13eDiborane(4) cations may have been involved in other C-H insertion events: H. Braunschweig, A. Damme, T. Kupfer, Chem. Commun. 2013, 49, 2774–2776.
- 14Gas-phase calculations indicate even stronger preference for the diborane(4) pathway. See the Supporting Information for details. As requested by a reviewer, calculations were also performed in PhBr solvent. In this case the diborane(4) pathway is still favored, although the difference between the two mechanisms is less prominent, and both may contribute in some cases. The majority of experimental work on high-temperature catalytic borylations, however, was performed in PhMe (see Refs. [2b,c]). Loss of the H2 byproduct from the reaction vessel is expected to further increase preference for the diborane(4) pathway.
- 15Several Me3P-based complex boron cations were reported previously (Refs. [9a, 17, 18]), thus justifying the choice of L for the initial studies.
- 16According to computational modeling, the cation 20 a is structurally similar to other species related to B3H8−. See the Supporting Information for details. The calculated 11B NMR chemical shifts of 20 a in PhBr (GIAO, SMD solvation) of δ=−9.3 and −40.9 ppm are within 2 ppm of the experimental values.
- 17M. Kameda, G. Kodama, J. Am. Chem. Soc. 1980, 102, 3647–3649.
- 18
- 18aR. DePoy, G. Kodama, Inorg. Chem. 1985, 24, 2871–2872;
- 18bR. E. DePoy, G. Kodama, Inorg. Chem. 1988, 27, 1116–1118;
- 18cR. E. DePoy, G. Kodama, Inorg. Chem. 1988, 27, 1836–1839.
- 19N. Schulenberg, H. Wadepohl, H.-J. Himmel, Angew. Chem. Int. Ed. 2011, 50, 10444–10447; Angew. Chem. 2011, 123, 10628–10631.
- 20The exact path for the formation of 19 was not investigated, although a role for trace L-BH3 appears plausible.
- 21L2B2H5+ (δ=11B −20.9 ppm) prepared from 1,3,4,5-tetramethylimidazolidene borane and 0.5 equiv Ph3C+ B(C6F5)4− in [D5]-PhBr upon heating to 90 °C yielded qualitatively similar results (δ=11B −7.2 (H-coupled, broad), −32.3 (t, JB-H=80 Hz), −34.2 ppm (H-coupled, broad)).
- 22A. Y. Bykov, K. Y. Zhizhin, N. T. Kuznetsov, Russ. J. Inorg. Chem. 2014, 59, 1539–1555.
- 23
- 23aH. Beall, D. F. Gaines, Inorg. Chim. Acta 1999, 289, 1–10;
- 23bS. Heřmánek, J. Plešek, Collect. Czech. Chem. Commun. 1966, 31, 177–189.
- 24
- 24aM. Krempp, R. Damrauer, C. H. DePuy, Y. Keheyan, J. Am. Chem. Soc. 1994, 116, 3629–3630;
- 24bR. C. Dunbar, J. Am. Chem. Soc. 1968, 90, 5676–5682.
- 25For a recent review of approaches to building electron-precise BB bonds, see: H. Braunschweig, R. D. Dewhurst, S. Mozo, ChemCatChem 2015, 7, 1630–1638.
- 26Attempts were made to prepare L2B2H4 complexes by reacting THF⋅B3H7 with tBuCH2NMe2, BnNMe2, tBuCH2PPh2, (o-Tol)3P, nBu3P, BnMeImd, and in all cases the desired neutral complex either did not form at all, or persisted in the reaction mixture only for a short time, before it could be isolated. This appears to be due to 1) Fast reaction between LB3H7 and L2B2H4 to produce LBH3 and higher boranes (M. Ishii, G. Kodama, Inorg. Chem. 1990, 29, 817–820) 2) Inherent instability of L2B2H4 complexes: (Me3P)2B2H4 decomposes at room temperature under N2 ( R. K. Hertz, M. L. Denniston, S. G. Shore, Inorg. Chem. 1978, 17, 2673–2674). Even (Ph3P)2B2H4, which is the most robust L2B2H4 complex because of its high crystallinity, decomposes at only 90 °C ( M. D. Levicheva, L. V. Titov, L. V. Zhemchugova, Izv. Akad. Nauk SSSR 1987, 2120–2122). In full agreement with literature reports, complexes with L=Me3N and Ph3P were prepared successfully, and therefore the process appears to be highly dependent on the nature of L. Reacting (Ph3P)2B2H4 with the N-heterocyclic carbene BnMeImd produced complex mixtures containing some of the desired L2B2H4 (δ=11B −34 ppm, br t) which could not be isolated, in agreement with the previous report by Curran et al. (Ref. [10b]).
- 27In view of the mechanism outlined in Figure 1, previously reported formation of L3B3H4+ cations in the electrophilic activation of L2B2H4 (Ref. [9a]) can now be interpreted as L2B2H3+ insertion into BB bond.
- 28No H2 was produced, thus ruling out the dehydrogenative pathway proposed above for salts with more stable anions. Involvement of L-BH2-H-B(C6F5)3 appears plausible based on limited NMR evidence.
- 29
- 29aDisproportionation between Me2SBH3 and B(C6F5)3: A.-M. Fuller, D. L. Hughes, S. J. Lancaster, C. M. White, Organometallics 2010, 29, 2194–2197;
- 29bC6F5-transfer in activated Me3NBH3: G. Ménard, D. W. Stephan, Dalton Trans. 2013, 42, 5447–5453.