Copper-Mediated Formation of Aryl, Heteroaryl, Vinyl and Alkynyl Difluoromethylphosphonates: A General Approach to Fluorinated Phosphate Mimics
Maria V. Ivanova
Normandie Univ., COBRA, UMR 6014 et FR 3038, Univ. Rouen, INSA Rouen, CNRS, 1 rue Tesnière, 76821 Mont Saint-Aignan Cedex (France)
These authors contributed equally to this work.
Search for more papers by this authorDr. Alexandre Bayle
Normandie Univ., COBRA, UMR 6014 et FR 3038, Univ. Rouen, INSA Rouen, CNRS, 1 rue Tesnière, 76821 Mont Saint-Aignan Cedex (France)
These authors contributed equally to this work.
Search for more papers by this authorDr. Tatiana Besset
Normandie Univ., COBRA, UMR 6014 et FR 3038, Univ. Rouen, INSA Rouen, CNRS, 1 rue Tesnière, 76821 Mont Saint-Aignan Cedex (France)
Search for more papers by this authorCorresponding Author
Dr. Thomas Poisson
Normandie Univ., COBRA, UMR 6014 et FR 3038, Univ. Rouen, INSA Rouen, CNRS, 1 rue Tesnière, 76821 Mont Saint-Aignan Cedex (France)
Normandie Univ., COBRA, UMR 6014 et FR 3038, Univ. Rouen, INSA Rouen, CNRS, 1 rue Tesnière, 76821 Mont Saint-Aignan Cedex (France)Search for more papers by this authorProf. Dr. Xavier Pannecoucke
Normandie Univ., COBRA, UMR 6014 et FR 3038, Univ. Rouen, INSA Rouen, CNRS, 1 rue Tesnière, 76821 Mont Saint-Aignan Cedex (France)
Search for more papers by this authorMaria V. Ivanova
Normandie Univ., COBRA, UMR 6014 et FR 3038, Univ. Rouen, INSA Rouen, CNRS, 1 rue Tesnière, 76821 Mont Saint-Aignan Cedex (France)
These authors contributed equally to this work.
Search for more papers by this authorDr. Alexandre Bayle
Normandie Univ., COBRA, UMR 6014 et FR 3038, Univ. Rouen, INSA Rouen, CNRS, 1 rue Tesnière, 76821 Mont Saint-Aignan Cedex (France)
These authors contributed equally to this work.
Search for more papers by this authorDr. Tatiana Besset
Normandie Univ., COBRA, UMR 6014 et FR 3038, Univ. Rouen, INSA Rouen, CNRS, 1 rue Tesnière, 76821 Mont Saint-Aignan Cedex (France)
Search for more papers by this authorCorresponding Author
Dr. Thomas Poisson
Normandie Univ., COBRA, UMR 6014 et FR 3038, Univ. Rouen, INSA Rouen, CNRS, 1 rue Tesnière, 76821 Mont Saint-Aignan Cedex (France)
Normandie Univ., COBRA, UMR 6014 et FR 3038, Univ. Rouen, INSA Rouen, CNRS, 1 rue Tesnière, 76821 Mont Saint-Aignan Cedex (France)Search for more papers by this authorProf. Dr. Xavier Pannecoucke
Normandie Univ., COBRA, UMR 6014 et FR 3038, Univ. Rouen, INSA Rouen, CNRS, 1 rue Tesnière, 76821 Mont Saint-Aignan Cedex (France)
Search for more papers by this authorGraphical Abstract
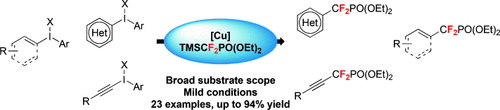
A general approach to aryl, heteroaryl, vinyl, and alkynyl difluoromethylphosphonates was developed providing access to a broad range of products including biologically relevant molecules in good to excellent yields. Mechanistic studies suggest the involvement of a copper(III) species as key intermediate.
Abstract
A general and efficient access to aryl, heteroaryl, vinyl and alkynyl difluoromethylphosphonates is described. The developed methodology using TMSCF2PO(OEt)2, iodonium salts and a copper salt provided a straightforward manifold to reach these highly relevant products. The reaction proved to be highly functional group tolerant and proceeded under mild conditions, giving the corresponding products in good to excellent yields. This method represents the first general synthetic route to this important class of fluorinated scaffolds, which are well-recognized as in vivo stable phosphate surrogates.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201507130_sm_miscellaneous_information.pdf3.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. O’Hagan, Chem. Soc. Rev. 2008, 37, 308–319;
- 1bR. D. Chambers, Fluorine in Organic Chemistry, Blackwell, Oxford, 2004;
10.1002/9781444305371 Google Scholar
- 1cK. Uneyama, Organofluorine Chemistry, Blackwell, Oxford, 2006.
10.1002/9780470988589 Google Scholar
- 2
- 2aS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320–330;
- 2bH.-J. Böhm, D. Banner, S. Bendels, M. Kansy, B. Kuhn, K. Müller, U. Obst-Sander, M. Stahl, ChemBioChem 2004, 5, 637–643;
- 2cJ. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok, H. Liu, Chem. Rev. 2014, 114, 2432–2506;
- 2dK. Müller, C. Faeh, F. Diederich, Science 2007, 317, 1881–1886;
- 2eE. A. Ilardi, E. Vitaku, J. T. Njardarson, J. Med. Chem. 2014, 57, 2832–2842.
- 3For selected reviews, see:
- 3aP. Chen, G. Liu, Synthesis 2013, 2919–2939;
- 3bH. Liu, Z. Gu, X. Jiang, Adv. Synth. Catal. 2013, 355, 617–626;
- 3cX.-F. Wu, H. Neumann, M. Beller, Chem. Asian J. 2012, 7, 1744–1754;
- 3dH. Egami, M. Sodeoka, Angew. Chem. Int. Ed. 2014, 53, 8294–8308; Angew. Chem. 2014, 126, 8434–8449;
- 3eE. Merino, C. Nevado, Chem. Soc. Rev. 2014, 43, 6598–6608;
- 3fT. Liang, C. N. Neumann, T. Ritter, Angew. Chem. Int. Ed. 2013, 52, 8214–8264; Angew. Chem. 2013, 125, 8372–8423;
- 3gG. Landelle, A. Panossian, S. Pazenok, J.-P. Vors, F. R. Leroux, Beilstein J. Org. Chem. 2013, 9, 2476–2536;
- 3hT. Besset, C. Schneider, D. Cahard, Angew. Chem. Int. Ed. 2012, 51, 5048–5050; Angew. Chem. 2012, 124, 5134–5136;
- 3iFor a special issue on fluorine chemistry, see: Chem. Rev. 2015, 115, 563–1306.
- 4
- 4aP. Xu, S. Guo, L. Wang, P. Tang, Synlett 2014, 36–39;
- 4bT. Besset, T. Poisson, X. Pannecoucke, Eur. J. Org. Chem. 2015, 2765–2789;
- 4cT. Besset, T. Poisson, X. Pannecoucke, Chem. Eur. J. 2014, 20, 16830–16845;
- 4dM.-C. Belhomme, T. Besset, T. Poisson, X. Pannecoucke, Chem. Eur. J. 2015, 21, 12836–12865;
- 4eC. Ni, L. Zhu, J. Hu, Acta Chim. Sin. 2015, 73, 90–115.
- 5aW. Howson, J. M. Hills, G. M. Blackburn, M. Broekman, Bioorg. Med. Chem. Lett. 1991, 1, 501–502;
- 5bG. M. Blackburn, D. E. Kent, F. Kolkmann, J. Chem. Soc. Chem. Commun. 1981, 1188–1190;
- 5cG. M. Blackburn, D. E. Kent, F. Kolkmann, J. Chem. Soc. Perkin Trans. 1 1984, 1119–1125;
- 5dT. R. Burke, M. S. Smyth, A. Otaka, M. Nomizu, P. P. Roller, G. Wolf, R. Case, S. E. Shoelson, Biochemistry 1994, 33, 6490–6494;
- 5eJ. X. Ong, C. W. Yap, W. H. Ang, Inorg. Chem. 2012, 51, 12483–12492.
- 6For selected examples, see:
- 6aD. Solas, R. L. Hale, D. V. Patel, J. Org. Chem. 1996, 61, 1537–1539;
- 6bI. G. Boutselis, X. Yu, Z.-Y. Zhang, R. F. Borch, J. Med. Chem. 2007, 50, 856–864;
- 6cG. Hum, J. Lee, S. D. Taylor, Bioorg. Med. Chem. Lett. 2002, 12, 3471–3474.
- 7aT. Yokomatsu, K. Suemune, T. Murano, S. Shibuya, J. Org. Chem. 1996, 61, 7207–7211;
- 7bX. Zhang, D. J. Burton, J. Fluorine Chem. 2002, 116, 15–18;
- 7cW. Qiu, D. J. Burton, Tetrahedron Lett. 1996, 37, 2745–2748;
- 7dX. Zhang, D. J. Burton, Tetrahedron Lett. 2000, 41, 7791–7794;
- 7eZ. Feng, F. Chen, X. Zhang, Org. Lett. 2012, 14, 1938–1941;
- 7fZ. Feng, Y.-L. Xiao, X. Zhang, Org. Chem. Front. 2014, 1, 113–116;
- 7gA. Bayle, C. Cocaud, C. Nicolas, O. R. Martin, T. Poisson, X. Pannecoucke, Eur. J. Org. Chem. 2015, 3787–3792.
- 8aZ. Feng, Q.-Q. Min, Y.-L. Xiao, B. Zhang, X. Zhang, Angew. Chem. Int. Ed. 2014, 53, 1669–1673; Angew. Chem. 2014, 126, 1695–1699;
- 8bX. Jiang, L. Chu, F.-L. Qing, New J. Chem. 2013, 37, 1736–1741.
- 9X. Jiang, L. Chu, F.-L. Qing, Org. Lett. 2012, 14, 2870–2873.
- 10
- 10aS. Murakami, H. Ishii, T. Tajima, T. Fuchigami, Tetrahedron 2006, 62, 3761–3769;
- 10bL. Wang, X.-J. Wei, W.-L. Lei, H. Chen, L.-Z. Wu, Q. Liu, Chem. Commun. 2014, 50, 15916–15919.
- 11
- 11aD. H. R. Barton, J. P. Finet, J. Khamsi, Tetrahedron Lett. 1988, 29, 1115–1118;
- 11bR. J. Phipps, N. P. Grimster, M. J. Gaunt, J. Am. Chem. Soc. 2008, 130, 8172–8174;
- 11cE. Cahard, N. Bremeyer, M. J. Gaunt, Angew. Chem. Int. Ed. 2013, 52, 9284–9288; Angew. Chem. 2013, 125, 9454–9458;
- 11dN. Gigant, L. Chausset-Boissarie, M.-C. Belhomme, T. Poisson, X. Pannecoucke, I. Gillaizeau, Org. Lett. 2013, 15, 278–281.
- 12Note that the use of unsymmetrical diaryl iodonium salts resulted in a mixture of products with no selectivity. Thus the use of a bulky mesitylene residue is of prime importance.
- 13When 1,10-phenanthroline was added to the competitive reaction between both iodonium salts a slight enhancement of the selectivity towards the formation of 3 e was observed (3 b:3 e=41:59) accompanied by a decrease of the reaction yield to 73 %.
- 14For selected examples involving the formation of a transient λ3-iodane, see:
- 14aR. Koller, K. Stanek, D. Stolz, R. Aardoom, K. Niedermann, A. Togni, Angew. Chem. Int. Ed. 2009, 48, 4332–4336; Angew. Chem. 2009, 121, 4396–4400;
- 14bA. E. Allen, D. W. C. MacMillan, J. Am. Chem. Soc. 2010, 132, 4986–4987;
- 14cK. Eastman, P. S. Baran, Tetrahedron 2009, 65, 3149–3154;
- 14dM. Ochiai, T. Shu, T. Nagaoka, Y. Kitagawa, J. Org. Chem. 1997, 62, 2130–2138;
- 14eJ. Malmgren, S. Santoro, N. Jalalian, F. Himo, B. Olofsson, Chem. Eur. J. 2013, 19, 10334–10342.