Supramolecular Assays for Mapping Enzyme Activity by Displacement-Triggered Change in Hyperpolarized 129Xe Magnetization Transfer NMR Spectroscopy
Dr. Matthias Schnurr
ERC Project BiosensorImaging, Leibniz-Institut für Molekulare Pharmakologie (FMP), Campus BerlinBuch, Robert-Rössle-Strasse 10, 13125 Berlin (Germany)
These authors contributed equally to this work.
Search for more papers by this authorDipl.-Chem. Jagoda Sloniec-Myszk
BAM Bundesanstalt für Materialforschung und -prüfung, Richard-Willstätter-Strasse 11, 12489 Berlin (Germany)
These authors contributed equally to this work.
Search for more papers by this authorDr. Jörg Döpfert
ERC Project BiosensorImaging, Leibniz-Institut für Molekulare Pharmakologie (FMP), Campus BerlinBuch, Robert-Rössle-Strasse 10, 13125 Berlin (Germany)
Search for more papers by this authorCorresponding Author
Dr. Leif Schröder
ERC Project BiosensorImaging, Leibniz-Institut für Molekulare Pharmakologie (FMP), Campus BerlinBuch, Robert-Rössle-Strasse 10, 13125 Berlin (Germany)
Leif Schröder, ERC Project BiosensorImaging, Leibniz-Institut für Molekulare Pharmakologie (FMP), Campus BerlinBuch, Robert-Rössle-Strasse 10, 13125 Berlin (Germany)
Andreas Hennig, Department of Life Sciences and Chemistry, Jacobs University Bremen, Campus Ring 1, 28759 Bremen (Germany)
Search for more papers by this authorCorresponding Author
Dr. Andreas Hennig
Department of Life Sciences and Chemistry, Jacobs University Bremen, Campus Ring 1, 28759 Bremen (Germany)
These authors contributed equally to this work.
Leif Schröder, ERC Project BiosensorImaging, Leibniz-Institut für Molekulare Pharmakologie (FMP), Campus BerlinBuch, Robert-Rössle-Strasse 10, 13125 Berlin (Germany)
Andreas Hennig, Department of Life Sciences and Chemistry, Jacobs University Bremen, Campus Ring 1, 28759 Bremen (Germany)
Search for more papers by this authorDr. Matthias Schnurr
ERC Project BiosensorImaging, Leibniz-Institut für Molekulare Pharmakologie (FMP), Campus BerlinBuch, Robert-Rössle-Strasse 10, 13125 Berlin (Germany)
These authors contributed equally to this work.
Search for more papers by this authorDipl.-Chem. Jagoda Sloniec-Myszk
BAM Bundesanstalt für Materialforschung und -prüfung, Richard-Willstätter-Strasse 11, 12489 Berlin (Germany)
These authors contributed equally to this work.
Search for more papers by this authorDr. Jörg Döpfert
ERC Project BiosensorImaging, Leibniz-Institut für Molekulare Pharmakologie (FMP), Campus BerlinBuch, Robert-Rössle-Strasse 10, 13125 Berlin (Germany)
Search for more papers by this authorCorresponding Author
Dr. Leif Schröder
ERC Project BiosensorImaging, Leibniz-Institut für Molekulare Pharmakologie (FMP), Campus BerlinBuch, Robert-Rössle-Strasse 10, 13125 Berlin (Germany)
Leif Schröder, ERC Project BiosensorImaging, Leibniz-Institut für Molekulare Pharmakologie (FMP), Campus BerlinBuch, Robert-Rössle-Strasse 10, 13125 Berlin (Germany)
Andreas Hennig, Department of Life Sciences and Chemistry, Jacobs University Bremen, Campus Ring 1, 28759 Bremen (Germany)
Search for more papers by this authorCorresponding Author
Dr. Andreas Hennig
Department of Life Sciences and Chemistry, Jacobs University Bremen, Campus Ring 1, 28759 Bremen (Germany)
These authors contributed equally to this work.
Leif Schröder, ERC Project BiosensorImaging, Leibniz-Institut für Molekulare Pharmakologie (FMP), Campus BerlinBuch, Robert-Rössle-Strasse 10, 13125 Berlin (Germany)
Andreas Hennig, Department of Life Sciences and Chemistry, Jacobs University Bremen, Campus Ring 1, 28759 Bremen (Germany)
Search for more papers by this authorGraphical Abstract
Abstract
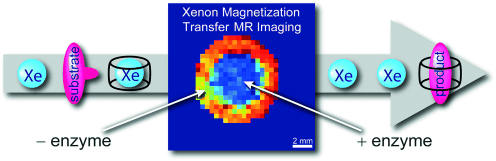
Reversibly bound Xe is a sensitive NMR and MRI reporter with its resonance frequency being influenced by the chemical environment of the host. Molecular imaging of enzyme activity presents a promising approach for disease identification, but current Xe biosensing concepts are limited since substrate conversion typically has little impact on the chemical shift of Xe inside tailored cavities. Herein, we exploit the ability of the product of the enzymatic reaction to bind itself to the macrocyclic hosts CB6 and CB7 and thereby displace Xe. We demonstrate the suitability of this method to map areas of enzyme activity through changes in magnetization transfer with hyperpolarized Xe under different saturation scenarios.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201507002_sm_miscellaneous_information.pdf1.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. K. Palaniappan, R. M. Ramirez, V. S. Bajaj, D. E. Wemmer, A. Pines, M. B. Francis, Angew. Chem. Int. Ed. 2013, 52, 4849–4853; Angew. Chem. 2013, 125, 4949–4953;
- 1bM. G. Shapiro, R. M. Ramirez, L. J. Sperling, G. Sun, J. Sun, A. Pines, D. V. Schaffer, V. S. Bajaj, Nat. Chem. 2014, 6, 629–634;
- 1cH. M. Rose, C. Witte, F. Rossella, S. Klippel, C. Freund, L. Schröder, Proc. Natl. Acad. Sci. USA 2014, 111, 11697–11702;
- 1dS. Klippel, C. Freund, L. Schröder, Nano Lett. 2014, 14, 5721–5726.
- 2
- 2aY.-Q. Song, B. M. Goodson, R. E. Taylor, D. D. Laws, G. Navon, A. Pines, Angew. Chem. Int. Ed. Engl. 1997, 36, 2368–2370; Angew. Chem. 1997, 109, 2464–2466;
- 2bK. Bartik, M. Luhmer, J.-P. Dutasta, A. Collet, J. Reisse, J. Am. Chem. Soc. 1998, 120, 784–791;
- 2cL. Schröder, T. J. Lowery, C. Hilty, D. E. Wemmer, A. Pines, Science 2006, 314, 446–449;
- 2dM. El Haouaj, M. Luhmer, Y. H. Ko, K. Kim, K. Bartik, J. Chem. Soc. Perkin Trans. 2 2001, 804–807;
- 2eM. E. Haouaj, Y. H. Ko, M. Luhmer, K. Kim, K. Bartik, J. Chem. Soc. Perkin Trans. 2 2001, 2104–2107;
- 2fB. S. Kim, Y. H. Ko, Y. Kim, H. J. Lee, N. Selvapalam, H. C. Lee, K. Kim, Chem. Commun. 2008, 2756–2758;
- 2gG. Huber, F.-X. Legrand, V. Lewin, D. Baumann, M.-P. Heck, P. Berthault, ChemPhysChem 2011, 12, 1053–1055;
- 2hT. Adiri, D. Marciano, Y. Cohen, Chem. Commun. 2013, 49, 7082–7084.
- 3
- 3aS. Klippel, J. Döpfert, J. Jayapaul, M. Kunth, F. Rossella, M. Schnurr, C. Witte, C. Freund, L. Schröder, Angew. Chem. Int. Ed. 2014, 53, 493–496; Angew. Chem. 2014, 126, 503–506;
- 3bM. Schnurr, K. Sydow, H. M. Rose, M. Dathe, L. Schröder, Adv. Healthcare Mater. 2015, 4, 40–45.
- 4
- 4aT. J. Lowery, S. Garcia, L. Chavez, E. J. Ruiz, T. Wu, T. Brotin, J.-P. Dutasta, D. S. King, P. G. Schultz, A. Pines, D. E. Wemmer, ChemBioChem 2006, 7, 65–73;
- 4bQ. Wei, G. K. Seward, P. A. Hill, B. Patton, I. E. Dimitrov, N. N. Kuzma, I. J. Dmochowski, J. Am. Chem. Soc. 2006, 128, 13274–13283;
- 4cV. Roy, T. Brotin, J.-P. Dutasta, M.-H. Charles, T. Delair, F. Mallet, G. Huber, H. Desvaux, Y. Boulard, P. Berthault, ChemPhysChem 2007, 8, 2082–2085;
- 4dJ. M. Chambers, P. A. Hill, J. A. Aaron, Z. Han, D. W. Christianson, N. N. Kuzma, I. J. Dmochowski, J. Am. Chem. Soc. 2009, 131, 563–569;
- 4eA. Schlundt, W. Kilian, M. Beyermann, J. Sticht, S. Günther, S. Höpner, K. Falk, O. Roetzschke, L. Mitschang, C. Freund, Angew. Chem. Int. Ed. 2009, 48, 4142–4145; Angew. Chem. 2009, 121, 4206–4209;
- 4fF. Schilling, L. Schröder, K. K. Palaniappan, S. Zapf, D. E. Wemmer, A. Pines, ChemPhysChem 2010, 11, 3529–3533;
- 4gG. K. Seward, Y. Bai, N. S. Khan, I. J. Dmochowski, Chem. Sci. 2011, 2, 1103–1110;
- 4hN. Kotera, N. Tassali, E. Léonce, C. Boutin, P. Berthault, T. Brotin, J.-P. Dutasta, L. Delacour, T. Traoré, D.-A. Buisson, F. Taran, S. Coudert, B. Rousseau, Angew. Chem. Int. Ed. 2012, 51, 4100–4103; Angew. Chem. 2012, 124, 4176–4179;
- 4iJ. Sloniec, M. Schnurr, C. Witte, U. Resch-Genger, L. Schröder, A. Hennig, Chem. Eur. J. 2013, 19, 3110–3118;
- 4jM. Schnurr, C. Witte, L. Schröder, Biophys. J. 2014, 106, 1301–1308;
- 4kM. Schnurr, C. Witte, L. Schröder, Phys. Chem. Chem. Phys. 2013, 15, 14178–14181.
- 5
- 5aR. Weissleder, M. J. Pittet, Nature 2008, 452, 580–589;
- 5bJ. Zhang, P. L. Yang, N. S. Gray, Nat. Rev. Cancer 2009, 9, 28–39;
- 5cB. De Strooper, R. Vassar, T. Golde, Nat. Rev. Neurol. 2010, 6, 99–107;
- 5dC. Lopez-Otin, T. Hunter, Nat. Rev. Cancer 2010, 10, 278–292;
- 5eK. Naylor, R. Eastell, Nat. Rev. Rheumatol. 2012, 8, 379–389.
- 6
- 6aJ. W. Lee, S. Samal, N. Selvapalam, H.-J. Kim, K. Kim, Acc. Chem. Res. 2003, 36, 621–630;
- 6bV. D. Uzunova, C. Cullinane, K. Brix, W. M. Nau, A. I. Day, Org. Biomol. Chem. 2010, 8, 2037–2042.
- 7
- 7aY. Wang, I. J. Dmochowski, Chem. Commun. 2015, 51, 8982–8985;
- 7bM. Kunth, C. Witte, A. Hennig, L. Schröder, Chem. Sci. 2015, DOI: 10.1039/C5SC01400J.
- 8
- 8aJ. Lagona, P. Mukhopadhyay, S. Chakrabarti, L. Isaacs, Angew. Chem. Int. Ed. 2005, 44, 4844–4870; Angew. Chem. 2005, 117, 4922–4949;
- 8bC. Kim, S. S. Agasti, Z. Zhu, L. Isaacs, V. M. Rotello, Nat. Chem. 2010, 2, 962–966;
- 8cI. Ghosh, W. M. Nau, Adv. Drug Delivery Rev. 2012, 64, 764–783.
- 9
- 9aA. Hennig, H. Bakirci, W. M. Nau, Nat. Methods 2007, 4, 629–632;
- 9bW. M. Nau, G. Ghale, A. Hennig, H. Bakirci, D. M. Bailey, J. Am. Chem. Soc. 2009, 131, 11558–11570;
- 9cR. N. Dsouza, A. Hennig, W. M. Nau, Chem. Eur. J. 2012, 18, 3444–3459;
- 9dA. Hennig in Supramolecular Systems in Biomedical Fields (Ed.: ), RSC, Cambridge, 2013, pp. 355–396;
10.1039/9781849737821-00355 Google Scholar
- 9eA. Praetorius, D. M. Bailey, T. Schwarzlose, W. M. Nau, Org. Lett. 2008, 10, 4089–4092;
- 9fG. Ghale, V. Ramalingam, A. R. Urbach, W. M. Nau, J. Am. Chem. Soc. 2011, 133, 7528–7535.
- 10E. W. Gerner, F. L. Meyskens, Nat. Rev. Cancer 2004, 4, 781–792.
- 11M. Florea, W. M. Nau, Angew. Chem. Int. Ed. 2011, 50, 9338–9342; Angew. Chem. 2011, 123, 9510–9514.
- 12The exact position of the peak depends on the composition of the buffer, in particular on the concentration of cations, such as Na+, which bind to the CB portals (see also Ref. [7]).
- 13R. M. Henkelman, X. Huang, Q.-S. Xiang, G. J. Stanisz, S. D. Swanson, M. J. Bronskill, Magn. Res. Med. 1993, 29, 759–766.
- 14L. Turati, M. Moscatelli, A. Mastropietro, N. G. Dowell, I. Zucca, A. Erbetta, C. Cordiglieri, G. Brenna, B. Bianchi, R. Mantegazza, M. Cercignani, F. Baggi, L. Minati, NMR Biomed. 2015, 28, 327–337.
- 15S. Aime, D. D. Castelli, E. Terreno, Angew. Chem. Int. Ed. 2002, 41, 4334–4336;
10.1002/1521-3773(20021115)41:22<4334::AID-ANIE4334>3.0.CO;2-1 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 4510–4512.
- 16A. Norouzy, Z. Azizi, W. M. Nau, Angew. Chem. Int. Ed. 2015, 54, 792–795; Angew. Chem. 2015, 127, 804–808.