Bacterial Synthesis of Unusual Sulfonamide and Sulfone Antibiotics by Flavoenzyme-Mediated Sulfur Dioxide Capture
Martin Baunach
Department of Biomolecular Chemistry, and Bio Pilot Plant, Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstrasse 11a, 07745 Jena (Germany)
These authors contributed equally to this work.
Search for more papers by this authorDr. Ling Ding
Department of Biomolecular Chemistry, and Bio Pilot Plant, Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstrasse 11a, 07745 Jena (Germany)
These authors contributed equally to this work.
Search for more papers by this authorKarsten Willing
Department of Biomolecular Chemistry, and Bio Pilot Plant, Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstrasse 11a, 07745 Jena (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Christian Hertweck
Department of Biomolecular Chemistry, and Bio Pilot Plant, Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstrasse 11a, 07745 Jena (Germany)
Chair for Natural Product Chemistry, Friedrich Schiller University, Jena (Germany)
Department of Biomolecular Chemistry, and Bio Pilot Plant, Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstrasse 11a, 07745 Jena (Germany)Search for more papers by this authorMartin Baunach
Department of Biomolecular Chemistry, and Bio Pilot Plant, Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstrasse 11a, 07745 Jena (Germany)
These authors contributed equally to this work.
Search for more papers by this authorDr. Ling Ding
Department of Biomolecular Chemistry, and Bio Pilot Plant, Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstrasse 11a, 07745 Jena (Germany)
These authors contributed equally to this work.
Search for more papers by this authorKarsten Willing
Department of Biomolecular Chemistry, and Bio Pilot Plant, Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstrasse 11a, 07745 Jena (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Christian Hertweck
Department of Biomolecular Chemistry, and Bio Pilot Plant, Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstrasse 11a, 07745 Jena (Germany)
Chair for Natural Product Chemistry, Friedrich Schiller University, Jena (Germany)
Department of Biomolecular Chemistry, and Bio Pilot Plant, Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstrasse 11a, 07745 Jena (Germany)Search for more papers by this authorGraphical Abstract
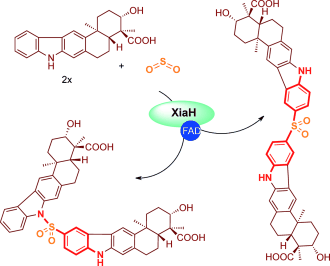
Ménage à trois: A radical-based, three-component reaction of xiamycin and sulfur dioxide leads to the biosynthesis of various bacterial sulfonamide and diarylsulfone antibiotics. Gene deletion, complementation, and biotransformation experiments unequivocally showed the involvement of the flavoprotein XiaH in the biosynthesis of these unprecedented sulfa compounds.
Abstract
Sulfa drugs, such as sulfonilamide and dapsone, are classical antibiotics that have been in clinical use worldwide. Despite the relatively simple architectures, practically no natural products are known to feature such aromatic sulfonamide or diarylsulfone substructures. We report the unexpected discovery of three fully unprecedented, sulfonyl-bridged alkaloid dimers (sulfadixiamycins A–C) from recombinant Streptomyces species harboring the entire xiamycin biosynthesis gene cluster. Sulfadixiamycins exhibit moderate antimycobacterial activities and potent antibiotic activities even against multidrug-resistant bacteria. Gene inactivation, complementation, and biotransformation experiments revealed that a flavin-dependent enzyme (XiaH) plays a key role in sulfadixiamycin biosynthesis. XiaH mediates a radical-based, three-component reaction involving two equivalents of xiamycin and sulfur dioxide, which is reminiscent of radical styrene/SO2 copolymerization.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201506541_sm_miscellaneous_information.pdf4.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. Bentley, J. Ind. Microbiol. Biotechnol. 2009, 36, 775–786.
- 2H. V. Iyer, J. Emerg. Med. 2008, 35, 209–210.
- 3E. Grundmann, Pathologe 2001, 22, 241–251.
- 4C. Capasso, C. T. Supuran, J. Enzyme Inhib. Med. Chem. 2014, 29, 379–387.
- 5
- 5aJ. Barr, J. Hist. Med. Allied Sci. 2011, 66, 425–467;
- 5bM. Rodriguez, J. A. Fishman, Clin. Microbiol. Rev. 2004, 17, 770–782;
- 5cH. K. Kar, R. Gupta, Clin. Dermatol. 2015, 33, 55–65.
- 6
- 6aK. K. Kim, J. G. Kang, S. S. Moon, K. Y. Kang, J. Antibiot. 2000, 53, 131–136;
- 6bS. Deng, S. N. Chen, P. Yao, D. Nikolic, R. B. van Breemen, J. L. Bolton, H. H. Fong, N. R. Farnsworth, G. F. Pauli, J. Nat. Prod. 2006, 69, 536–541;
- 6cR. Pozzi, M. Simone, C. Mazzetti, S. Maffioli, P. Monciardini, L. Cavaletti, R. Bamonte, M. Sosio, S. Donadio, J. Antibiot. 2011, 64, 133–139.
- 7S. P. B. Ovenden, R. J. Capon, J. Nat. Prod. 1999, 62, 1246–1249.
- 8
- 8aL. Ding, J. Munch, H. Goerls, A. Maier, H. H. Fiebig, W. H. Lin, C. Hertweck, Bioorg. Med. Chem. Lett. 2010, 20, 6685–6687;
- 8bL. Ding, A. Maier, H. H. Fiebig, W. H. Lin, C. Hertweck, Org. Biomol. Chem. 2011, 9, 4029–4031.
- 9
- 9aZ. Xu, M. Baunach, L. Ding, C. Hertweck, Angew. Chem. Int. Ed. 2012, 51, 10293–10297; Angew. Chem. 2012, 124, 10439–10443;
- 9bH. Li, Q. Zhang, S. Li, Y. Zhu, G. Zhang, H. Zhang, X. Tian, S. Zhang, J. Ju, C. Zhang, J. Am. Chem. Soc. 2012, 134, 8996–9005;
- 9cY. Sun, P. Chen, D. Zhang, M. Baunach, C. Hertweck, A. Li, Angew. Chem. Int. Ed. 2014, 53, 9012–9016; Angew. Chem. 2014, 126, 9158–9162;
- 9dH. Li, Y. Sun, Q. Zhang, Y. Zhu, S. M. Li, A. Li, C. Zhang, Org. Lett. 2015, 17, 306–309;
- 9eM. Baunach, J. Franke, C. Hertweck, Angew. Chem. Int. Ed. 2015, 54, 2604–2626; Angew. Chem. 2015, 127, 2640–2664.
- 10M. Baunach, L. Ding, T. Bruhn, G. Bringmann, C. Hertweck, Angew. Chem. Int. Ed. 2013, 52, 9040–9043; Angew. Chem. 2013, 125, 9210–9213.
- 11
- 11aM. Horinouchi, K. Kasuga, H. Nojiri, H. Yamane, T. Omori, FEMS Microbiol. Lett. 1997, 155, 99–105;
- 11bX. Zhou, X. Chen, Y. Jin, I. E. Markó, Chem. Asian J. 2012, 7, 2253–2257.
- 12
- 12aW. G. Barb, Proc. R. Soc. London Ser. A 1952, 212, 66-80;
- 12bM. Matsuda, M. Iino, T. Hirayama, T. Miyashita, Macromolecules 1972, 5, 240–246.
- 13M. Fischer, C. Schmidt, D. Falke, R. G. Sawers, Res. Microbiol. 2012, 163, 340–348.
- 14B. R. Rosen, E. W. Werner, A. G. O′Brien, P. S. Baran, J. Am. Chem. Soc. 2014, 136, 5571–5574.
- 15
- 15aJ. F. Ambrose, L. L. Carpenter, R. F. Nelson, J. Electrochem. Soc. 1975, 122, 876–894;
- 15bC. Berti, L. Greci, R. Andruzzi, A. Trazza, J. Org. Chem. 1985, 50, 368–373.
- 16
- 16aD. Zheng, Y. An, Z. Li, J. Wu, Angew. Chem. Int. Ed. 2014, 53, 2451–2454; Angew. Chem. 2014, 126, 2483–2486;
- 16bG. Liu, C. Fan, J. Wu, Org. Biomol. Chem. 2015, 13, 1592–1599.
- 17C. T. Walsh, T. A. Wencewicz, Nat. Prod. Rep. 2013, 30, 175–200.
- 18R. M. S. E. Rigby, R. M. Hynson, R. R. Ramsay, A. W. Munro, N. S. Scrutton, J. Biol. Chem. 2005, 280, 4627–4631.
- 19K. Yamanaka, K. S. Ryan, T. A. Gulder, C. C. Hughes, B. S. Moore, J. Am. Chem. Soc. 2012, 134, 12434–12437.
- 20
- 20aB. Li, J. P. Yu, J. S. Brunzelle, G. N. Moll, W. A. van der Donk, S. K. Nair, Science 2006, 311, 1464–1467;
- 20bA. Braunshausen, F. P. Seebeck, J. Am. Chem. Soc. 2011, 133, 1757–1759;
- 20cD. H. Scharf, N. Remme, A. Habel, P. Chankhamjon, K. Scherlach, T. Heinekamp, P. Hortschansky, A. A. Brakhage, C. Hertweck, J. Am. Chem. Soc. 2011, 133, 12322–12325;
- 20dD. H. Scharf, P. Chankhamjon, K. Scherlach, T. Heinekamp, K. Willing, A. A. Brakhage, C. Hertweck, Angew. Chem. Int. Ed. 2013, 52, 11092–11095; Angew. Chem. 2013, 125, 11298–11301;
- 20eS. Coyne, C. Chizzali, M. N. Khalil, A. Litomska, K. Richter, L. Beerhues, C. Hertweck, Angew. Chem. Int. Ed. 2013, 52, 10564–10568; Angew. Chem. 2013, 125, 10758–10762;
- 20fE. Sasaki, X. Zhang, H. G. Sun, M. Y. Lu, T. L. Liu, A. Ou, J. Y. Li, Y. H. Chen, S. E. Ealick, H. W. Liu, Nature 2014, 510, 427–431;
- 20gK. V. Goncharenko, A. Vit, W. Blankenfeldt, F. P. Seebeck, Angew. Chem. Int. Ed. 2015, 54, 2821–2824; Angew. Chem. 2015, 127, 2863–2866.
- 21H. Aldemir, R. Richarz, T. A. Gulder, Angew. Chem. Int. Ed. 2014, 53, 8286–8293; Angew. Chem. 2014, 126, 8426–8433.