Biocatalytic Feedback-Driven Temporal Programming of Self-Regulating Peptide Hydrogels
Thomas Heuser
DWI—Leibniz Institute for Interactive Materials, Forckenbeckstrasse 50, 52056 Aachen (Germany) http://www.dwi.rwth-aachen.de
Search for more papers by this authorElisabeth Weyandt
DWI—Leibniz Institute for Interactive Materials, Forckenbeckstrasse 50, 52056 Aachen (Germany) http://www.dwi.rwth-aachen.de
Search for more papers by this authorCorresponding Author
Dr. Andreas Walther
DWI—Leibniz Institute for Interactive Materials, Forckenbeckstrasse 50, 52056 Aachen (Germany) http://www.dwi.rwth-aachen.de
DWI—Leibniz Institute for Interactive Materials, Forckenbeckstrasse 50, 52056 Aachen (Germany) http://www.dwi.rwth-aachen.deSearch for more papers by this authorThomas Heuser
DWI—Leibniz Institute for Interactive Materials, Forckenbeckstrasse 50, 52056 Aachen (Germany) http://www.dwi.rwth-aachen.de
Search for more papers by this authorElisabeth Weyandt
DWI—Leibniz Institute for Interactive Materials, Forckenbeckstrasse 50, 52056 Aachen (Germany) http://www.dwi.rwth-aachen.de
Search for more papers by this authorCorresponding Author
Dr. Andreas Walther
DWI—Leibniz Institute for Interactive Materials, Forckenbeckstrasse 50, 52056 Aachen (Germany) http://www.dwi.rwth-aachen.de
DWI—Leibniz Institute for Interactive Materials, Forckenbeckstrasse 50, 52056 Aachen (Germany) http://www.dwi.rwth-aachen.deSearch for more papers by this authorGraphical Abstract
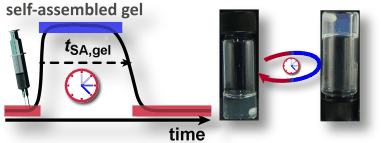
Programmed to self-destruct: An internal enzymatic feedback system enables the autonomous self-regulation over time of a pH-responsive peptide gelator (see picture; SA=self-assembly). The resulting dynamic hydrogels with programmed lifetimes are suitable for application in fluidic guidance, burst release, and transient rapid prototyping.
Abstract
Switchable self-assemblies respond to external stimuli with a transition between near-equilibrium states. Although being a key to present-day advanced materials, these systems respond rather passively, and do not display autonomous dynamics. For autonomous behavior, approaches must be found to orchestrate the time domain of self-assemblies, which would lead to new generations of dynamic and self-regulating materials. Herein, we demonstrate catalytic control of the time domain of pH-responsive peptide hydrogelators in a closed system. We program transient acidic pH states by combining a fast acidic activator with the slow, enzymatic, feedback-driven generation of a base (dormant deactivator). This transient state can be programmed over orders of magnitude in time. It is coupled to dipeptides to create autonomously self-regulating, dynamic gels with programmed lifetimes, which are used for fluidic guidance, burst release, and self-erasing rapid prototyping.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201505013_sm_MovieS1.mp44.7 MB | MovieS1 |
| anie_201505013_sm_MovieS2.mp45.8 MB | MovieS2 |
| anie_201505013_sm_miscellaneous_information.pdf950 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. Stoffelen, J. Voskuhl, P. Jonkheijm, J. Huskens, Angew. Chem. Int. Ed. 2014, 53, 3400–3404; Angew. Chem. 2014, 126, 3468–3472;
- 1bL. Chen, K. Morris, A. Laybourn, D. Elias, M. R. Hicks, A. Rodger, L. Serpell, D. J. Adams, Langmuir 2010, 26, 5232–5242.
- 2
- 2aY. Kang, J. J. Walish, T. Gorishnyy, E. L. Thomas, Nat. Mater. 2007, 6, 957–960;
- 2bA. C. Arsenault, D. P. Puzzo, I. Manners, G. A. Ozin, Nat. Photonics 2007, 1, 468–472.
- 3M. Fidaleo, R. Lavecchia, Chem. Biochem. Eng. Q. 2003, 17, 311–318.
- 4
- 4aC. T. Huynh, M. K. Nguyen, D. S. Lee, Macromolecules 2011, 44, 6629–6636;
- 4bS. Yan, T. Wang, L. Feng, J. Zhu, K. Zhang, X. Chen, L. Cui, J. Yin, Biomacromolecules 2014, 15, 4495–4508;
- 4cS. Zhang, M. A. Greenfield, A. Mata, L. C. Palmer, R. Bitton, J. R. Mantei, C. Aparicio, M. O. de La Cruz, S. I. Stupp, Nat. Mater. 2010, 9, 594–601;
- 4dJ.-F. Lutz, H. G. Börner, Prog. Polym. Sci. 2008, 33, 1–39.
- 5
- 5aW. T. S. Huck, Mater. Today 2008, 11, 24–32;
- 5bS. Moya, O. Azzaroni, T. Farhan, V. L. Osborne, W. T. S. Huck, Angew. Chem. Int. Ed. 2005, 44, 4578–4581; Angew. Chem. 2005, 117, 4654–4657.
- 6
- 6aY. Zhu, J. Shi, W. Shen, X. Dong, J. Feng, M. Ruan, Y. Li, Angew. Chem. Int. Ed. 2005, 44, 5083–5087; Angew. Chem. 2005, 117, 5213–5217;
- 6bY. Bae, S. Fukushima, A. Harada, K. Kataoka, Angew. Chem. Int. Ed. 2003, 42, 4640–4643; Angew. Chem. 2003, 115, 4788–4791.
- 7
- 7aY. Li, B. S. Lokitz, C. L. McCormick, Angew. Chem. Int. Ed. 2006, 45, 5792–5795; Angew. Chem. 2006, 118, 5924–5927;
- 7bM. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov, S. Minko, Nat. Mater. 2010, 9, 101–113.
- 8
- 8aD. A. Fletcher, R. D. Mullins, Nature 2010, 463, 485–492;
- 8bT. Mitchison, M. Kirschner, Nature 1984, 312, 237–242;
- 8cG. M. Whitesides, B. Grzybowski, Science 2002, 295, 2418–2421.
- 9
- 9aC. Wang, R. J. Stewart, J. Kopecek, Nature 1999, 397, 417–420;
- 9bA. M. Smith, R. J. Williams, C. Tang, P. Coppo, R. F. Collins, M. L. Turner, A. Saiani, R. V. Ulijn, Adv. Mater. 2008, 20, 37–41;
- 9cA. Aggeli, M. Bell, N. Boden, L. M. Carrick, A. E. Strong, Angew. Chem. Int. Ed. 2003, 42, 5603–5606; Angew. Chem. 2003, 115, 5761–5764.
- 10
- 10aS. Shinohara, T. Seki, T. Sakai, R. Yoshida, Y. Takeoka, Angew. Chem. Int. Ed. 2008, 47, 9039–9043; Angew. Chem. 2008, 120, 9179–9183;
- 10bS. Maeda, Y. Hara, R. Yoshida, S. Hashimoto, Angew. Chem. Int. Ed. 2008, 47, 6690–6693; Angew. Chem. 2008, 120, 6792–6795;
- 10cS. Maeda, Y. Hara, T. Sakai, R. Yoshida, S. Hashimoto, Adv. Mater. 2007, 19, 3480–3484.
- 11S. N. Semenov, A. S. Y. Wong, R. M. van der Made, S. G. J. Postma, J. Groen, H. W. H. van Roekel, T. F. A. de Greef, W. T. S. Huck, Nat. Chem. 2015, 7, 160–165.
- 12
- 12aS. Debnath, S. Roy, R. V. Ulijn, J. Am. Chem. Soc. 2013, 135, 16789–16792;
- 12bC. G. Pappas, I. R. Sasselli, R. V. Ulijn, Angew. Chem. Int. Ed. 2015, 54, 8119–8123; Angew. Chem. 2015, 127, 8237–8241.
- 13J. Boekhoven, A. M. Brizard, K. N. K. Kowlgi, G. J. M. Koper, R. Eelkema, J. H. van Esch, Angew. Chem. Int. Ed. 2010, 49, 4825–4828; Angew. Chem. 2010, 122, 4935–4938.
- 14T. Heuser, A.-K. Steppert, C. Molano Lopez, B. Zhu, A. Walther, Nano Lett. 2015, 15, 2213–2219.
- 15G. B. Kistiakowsky, A. J. Rosenberg, J. Am. Chem. Soc. 1952, 74, 5020–5025.
- 16A. J. Russell, M. Erbeldinger, J. J. DeFrank, J. Kaar, G. Drevon, Biotechnol. Bioeng. 2002, 77, 352–357.
- 17
- 17aG. Hu, J. A. Pojman, S. K. Scott, M. M. Wrobel, A. F. Taylor, J. Phys. Chem. B 2010, 114, 14059–14063;
- 17bJ. M. Johnson, N. Kinsinger, C. Sun, D. Li, D. Kisailus, J. Am. Chem. Soc. 2012, 134, 13974–13977;
- 17cR. de La Rica, H. Matsui, Angew. Chem. Int. Ed. 2008, 47, 5415–5417; Angew. Chem. 2008, 120, 5495–5497.
- 18T.-C. Huang, D.-H. Chen, J. Chem. Technol. Biotechnol. 1991, 52, 433–444.
- 19B. Krajewska, J. Mol. Catal. B 2009, 59, 9–21.
- 20C. Tang, R. V. Ulijn, A. Saiani, Langmuir 2011, 27, 14438–14449.
- 21
- 21aA. J. deMello, Nature 2006, 442, 394–402;
- 21bM. Prakash, N. Gershenfeld, Science 2007, 315, 832–835.
- 22A. Lenshof, T. Laurell, Chem. Soc. Rev. 2010, 39, 1203–1217.