Rhodium-Catalyzed [3+2+2] and [2+2+2] Cycloadditions of Two Alkynes with Cyclopropylideneacetamides†
Tomoka Yoshida
Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588 (Japan)
Search for more papers by this authorYuki Tajima
Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588 (Japan)
Search for more papers by this authorMasayuki Kobayashi
Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588 (Japan)
Search for more papers by this authorKoji Masutomi
Department of Applied Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology, Ookayama, Meguro-ku, Tokyo 152-8550 (Japan) http://www.apc.titech.ac.jp/∼ktanaka/
Search for more papers by this authorProf. Dr. Keiichi Noguchi
Instrumentation Analysis Center, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Ken Tanaka
Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588 (Japan)
Department of Applied Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology, Ookayama, Meguro-ku, Tokyo 152-8550 (Japan) http://www.apc.titech.ac.jp/∼ktanaka/
Japan Science and Technology Agency (JST), ACT-C, 4-1-8 Honcho, Kawaguchi, Saitama, 332-0012 (Japan)
Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588 (Japan)Search for more papers by this authorTomoka Yoshida
Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588 (Japan)
Search for more papers by this authorYuki Tajima
Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588 (Japan)
Search for more papers by this authorMasayuki Kobayashi
Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588 (Japan)
Search for more papers by this authorKoji Masutomi
Department of Applied Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology, Ookayama, Meguro-ku, Tokyo 152-8550 (Japan) http://www.apc.titech.ac.jp/∼ktanaka/
Search for more papers by this authorProf. Dr. Keiichi Noguchi
Instrumentation Analysis Center, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Ken Tanaka
Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588 (Japan)
Department of Applied Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology, Ookayama, Meguro-ku, Tokyo 152-8550 (Japan) http://www.apc.titech.ac.jp/∼ktanaka/
Japan Science and Technology Agency (JST), ACT-C, 4-1-8 Honcho, Kawaguchi, Saitama, 332-0012 (Japan)
Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588 (Japan)Search for more papers by this authorThis work was supported partly by a Grant-in-Aid for Scientific Research (No 25105714) from the the Ministry of Education, Culture, Sports, Science and Technology [MEXT (Japan)] and by ACT-C from the Japan Science and Technology Agency ]JST (Japan)]. We thank Takasago International Corporation for the gift of segphos and H8-binap, Umicore for generous support in supplying rhodium complexes, and Dr. Yu Shibata (Tokyo Institute of Technology) for assistance in the preparation of the manuscript.
Graphical Abstract
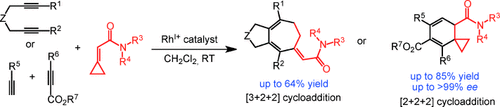
‘Cat nap’: The cationic RhI/H8-binap complex catalyzes the [3+2+2] cycloaddition of 1,6-diynes with cyclopropylideneacetamides to produce cycloheptadienes. In contrast, the cationic RhI/(S)-binap complex catalyzes the enantioselective [2+2+2] cycloaddition of terminal alkynes, acetylenedicarboxylates, and cyclopropylideneacetamides to produce spiro-cyclohexadienes.
Abstract
It has been established that a cationic rhodium(I)/H8-binap complex catalyzes the [3+2+2] cycloaddition of 1,6-diynes with cyclopropylideneacetamides to produce cycloheptadiene derivatives through cleavage of cyclopropane rings. In contrast, a cationic rhodium(I)/(S)-binap complex catalyzes the enantioselective [2+2+2] cycloaddition of terminal alkynes, acetylenedicarboxylates, and cyclopropylideneacetamides to produce spiro-cyclohexadiene derivatives which retain the cyclopropane rings.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201502505_sm_miscellaneous_information.pdf6.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aP. A. Inglesby, P. A. Evans in Comprehensive Organic Synthesis II, Vol. 5 (Eds.: ), Elsevier, Amsterdam, 2014, p. 656;
10.1016/B978-0-08-097742-3.00515-2 Google Scholar
- 1bA. Brandi, S. Cicchi, F. M. Cordero, A. Goti, Chem. Rev. 2014, 114, 7317;
- 1cH. Pellissier, Tetrahedron 2014, 70, 4991;
- 1dL. Yu, R. Guo, Org. Prep. Proced. Int. 2011, 43, 209;
- 1eM. Gulias, F. Lopez, J. L. Mascarenas, Pure Appl. Chem. 2011, 83, 495;
- 1fH. Pellissier, Tetrahedron 2010, 66, 8341.
- 2For the [3+2+2] cycloadditions of two alkynes with the cyclopropylideneacetate, see:
- 2aR. Yamasaki, N. Terashima, I. Sotome, S. Komagawa, S. Saito, J. Org. Chem. 2010, 75, 480;
- 2bR. A. Bauer, C. M. DiBlasi, D. S. Tan, Org. Lett. 2010, 12, 2084;
- 2cS. Komagawa, K. Takeuchi, I. Sotome, I. Azumaya, H. Masu, R. Yamasaki, S. Saito, J. Org. Chem. 2009, 74, 3323;
- 2dR. Yamasaki, I. Sotome, S. Komagawa, I. Azumaya, H. Masu, S. Saito, Tetrahedron Lett. 2009, 50, 1143;
- 2eK. Maeda, S. Saito, Tetrahedron Lett. 2007, 48, 3173;
- 2fS. Saito, S. Komagawa, I. Azumaya, M. Masuda, J. Org. Chem. 2007, 72, 9114;
- 2gS. Komagawa, S. Saito, Angew. Chem. Int. Ed. 2006, 45, 2446; Angew. Chem. 2006, 118, 2506;
- 2hS. Saito, M. Masuda, S. Komagawa, J. Am. Chem. Soc. 2004, 126, 10540.
- 3For the cycloadditions involving the cyclopropylideneacetate, see:
- 3aR. Yamasaki, M. Ohashi, K. Maeda, T. Kitamura, M. Nakagawa, K. Kato, T. Fujita, R. Kamura, K. Kinoshita, H. Masu, I. Azumaya, S. Ogoshi, S. Saito, Chem. Eur. J. 2013, 19, 3415;
- 3bR. Yamasaki, K. Kato, D. Hanitani, Y. Mutoh, S. Saito, Tetrahedron Lett. 2013, 54, 3507;
- 3cM. Ohashi, T. Taniguchi, S. Ogoshi, J. Am. Chem. Soc. 2011, 133, 14900;
- 3dS. Saito, K. Maeda, R. Yamasaki, T. Kitamura, M. Nakagawa, K. Kato, I. Azumaya, H. Masu, Angew. Chem. Int. Ed. 2010, 49, 1830; Angew. Chem. 2010, 122, 1874;
- 3eS. Saito, T. Yoshizawa, S. Ishigami, R. Yamasaki, Tetrahedron Lett. 2010, 51, 6028;
- 3fM. Ohashi, T. Taniguchi, S. Ogoshi, Organometallics 2010, 29, 2386;
- 3gS. Saito, K. Takeuchi, Tetrahedron Lett. 2007, 48, 595;
- 3hT. Kawasaki, S. Saito, Y. Yamamoto, J. Org. Chem. 2002, 67, 4911.
- 4For detailed mechanistic studies, see:
- 4aS. Komagawa, C. Wang, K. Morokuma, S. Saito, M. Uchiyama, J. Am. Chem. Soc. 2013, 135, 14508;
- 4bY. An, C. Cheng, B. Pan, Z. Wang, Eur. J. Org. Chem. 2012, 3911.
- 5For the [2+2+2] cycloaddition reactions involving cyclopropylidene units which retain cyclopropane rings, see:
- 5aG. Bhargava, B. Trillo, M. Araya, F. Lopez, L. Castedo, J. L. Mascarenas, Chem. Commun. 2010, 46, 270;
- 5bL. Zhao, A. de Meijere, Adv. Synth. Catal. 2006, 348, 2484;
- 5cM. Schelper, O. Buisine, S. Kozhushkov, C. Aubert, A. de Meijere, M. Malacria, Eur. J. Org. Chem. 2005, 3000.
- 6G. Vidari, P. Vita-Finzi in Studies in Natural Products Chemistry, Structure and Chemistry, Vol. 17 (Ed.: ), Elsevier, Amsterdam, 1995, p. 153.
- 7
- 7aD. S. Siegel, G. Piizzi, G. Piersanti, M. Movassaghi, J. Org. Chem. 2009, 74, 9292;
- 7bM. Movassaghi, G. Piizzi, D. S. Siegel, G. Piersanti, Angew. Chem. Int. Ed. 2006, 45, 5859; Angew. Chem. 2006, 118, 5991.
- 8For reviews, see:
- 8aK. Tanaka, Transition-Metal-Mediated Aromatic Ring Construction (Ed.: ), Wiley, Hoboken, 2013, chap. 4;
- 8bY. Shibata, K. Tanaka, Synthesis 2012, 44, 323;
- 8cK. Tanaka, Heterocycles 2012, 85, 1017;
- 8dK. Tanaka, Chem. Asian J. 2009, 4, 508;
- 8eK. Tanaka, Synlett 2007, 1977.
- 9For initial works, see:
- 9aK. Tanaka, K. Shirasaka, Org. Lett. 2003, 5, 4697;
- 9bK. Tanaka, K. Toyoda, A. Wada, K. Shirasaka, M. Hirano, Chem. Eur. J. 2005, 11, 1145.
- 10For the rhodium(I)-catalyzed [3+2+2] cycloaddition involving cyclopropylidene compounds, see:
- 10aP. A. Evans, D. E. Negru, D. Shang, Angew. Chem. Int. Ed. 2015, 127, 4850; Angew. Chem. 2015, 54, 4768;
- 10bM. Araya, M. Gulías, I. Fernández, G. Bhargava, L. Castedo, J. L. Mascareñas, Chem. Eur. J. 2014, 20, 10255;
- 10cP. A. Inglesby, J. Bacsa, D. E. Negru, P. A. Evans, Angew. Chem. Int. Ed. 2014, 53, 3952; Angew. Chem. 2014, 126, 4033;
- 10dP. A. Evans, T. Baikstis, P. A. Inglesby, Tetrahedron 2013, 69, 7826;
- 10eH. Yu, Q. Lu, Z. Dang, Y. Fu, Chem. Asian J. 2013, 8, 2262;
- 10fP. A. Evans, P. A. Inglesby, K. Kilbride, Org. Lett. 2013, 15, 1798;
- 10gP. A. Evans, P. A. Inglesby, J. Am. Chem. Soc. 2008, 130, 12838.
- 11For examples of the cationic rhodium(I)-complex-catalyzed [2+2+2] cycloaddition involving acrylamide derivatives, see:
- 11aJ. Hara, M. Ishida, M. Kobayashi, K. Noguchi, K. Tanaka, Angew. Chem. Int. Ed. 2014, 53, 2956; Angew. Chem. 2014, 126, 3000;
- 11bK. Masutomi, N. Sakiyama, K. Noguchi, K. Tanaka, Angew. Chem. Int. Ed. 2012, 51, 13031; Angew. Chem. 2012, 124, 13208;
- 11cM. Kobayashi, T. Suda, K. Noguchi, K. Tanaka, Angew. Chem. Int. Ed. 2011, 50, 1664; Angew. Chem. 2011, 123, 1702;
- 11dK. Tanaka, M. Takahashi, H. Imase, T. Osaka, K. Noguchi, M. Hirano, Tetrahedron 2008, 64, 6289.
- 12M. Kobayashi, K. Tanaka, Chem. Eur. J. 2012, 18, 9225.
- 13See the Supporting Information for details.
- 14CCDC 1052734 [(−)-8 kac] and 1052739 [(+)-10] contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15J. Izquierdo, S. Rodríguez, F. V. González, Org. Lett. 2011, 13, 3856.
- 16
- 16aSee Ref. [9b];
- 16bK. Tanaka, N. Suzuki, G. Nishida, Eur. J. Org. Chem. 2006, 3917.