Cyclic Hypervalent Iodine Reagents and Iron Catalysts: The Winning Team for Late-Stage CH Azidation†
We thank the Swiss National Science Foundation (SNSF; 200020_134550) for supporting M.V.V. and Dr. Fides Benfatti and Dr. Tony O’Sullivan from Syngenta Crop Protection Münchwilen AG for providing DSC measurements.
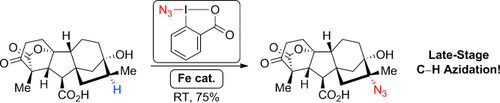
Graphical Abstract
1+1=3: By combining the exceptional reactivities of cyclic hypervalent iodine reagents and iron catalysts, Sharma and Hartwig achieved the azidation of CH bonds with unprecedented efficiency and selectivity. The late-stage introduction of azides into complex bioactive molecules will greatly facilitate the synthesis of analogues and accelerate the discovery of new chemical entities.