The Synthesis and Biological Evaluation of Desepoxyisotedanolide and a Comparison with Desepoxytedanolide†
We thank Prof. Daniel Romo, Department of Chemistry, Texas A&M University for a generous gift of DMDA-pateamine A, Bettina Hinkelmann, HZI, for skilful technical assistance and gratefully acknowledge funding by the DFG (KA 913/17-1, SA 356/7-1). RET acknowledges funding support from the National Science Foundation (CHE-0924351).
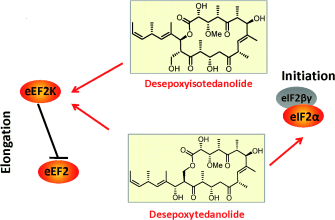
Graphical Abstract
A step in the right direction: Desepoxyisotedanolide, a hypothetical biosynthetic precursor of the tedanolide desepoxytedanolide, was synthesized. Biological studies of the two macrolactones revealed an additional cellular target for desepoxytedanolide, as well as evidence that the proposed isomerization of the precursor provides a survival advantage for the producing microorganism.
Abstract
The tedanolides are biologically active polyketides that exhibit a macrolactone constructed from a primary alcohol. Since polyketidal transformations only generate secondary alcohols, it has been hypothesized by Taylor that this unique lactone could arise from a postketidal transesterification. In order to probe this hypothesis and to investigate the biological profile of the putative precursor of all members of the tedanolide family, we embarked on the synthesis of desepoxyisotedanolide and its biological evaluation in comparison to desepoxytedanolide. The biological experiments unraveled a second target for desepoxytedanolide and provided evidence that the proposed transesterification indeed provides a survival advantage for the producing microorganism.