Organocatalyzed CO2 Trapping Using Alkynyl Indoles†
Dr. Zhuo Xin
Carbon Dioxide Activation Center (CADIAC), Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus (Denmark) http://www.skrydstrup-group.com
Search for more papers by this authorDr. Camille Lescot
Carbon Dioxide Activation Center (CADIAC), Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus (Denmark) http://www.skrydstrup-group.com
Search for more papers by this authorDr. Stig D. Friis
Carbon Dioxide Activation Center (CADIAC), Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus (Denmark) http://www.skrydstrup-group.com
Search for more papers by this authorProf. Dr. Kim Daasbjerg
Carbon Dioxide Activation Center (CADIAC), Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus (Denmark) http://www.skrydstrup-group.com
Search for more papers by this authorCorresponding Author
Prof. Dr. Troels Skrydstrup
Carbon Dioxide Activation Center (CADIAC), Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus (Denmark) http://www.skrydstrup-group.com
Carbon Dioxide Activation Center (CADIAC), Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus (Denmark) http://www.skrydstrup-group.comSearch for more papers by this authorDr. Zhuo Xin
Carbon Dioxide Activation Center (CADIAC), Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus (Denmark) http://www.skrydstrup-group.com
Search for more papers by this authorDr. Camille Lescot
Carbon Dioxide Activation Center (CADIAC), Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus (Denmark) http://www.skrydstrup-group.com
Search for more papers by this authorDr. Stig D. Friis
Carbon Dioxide Activation Center (CADIAC), Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus (Denmark) http://www.skrydstrup-group.com
Search for more papers by this authorProf. Dr. Kim Daasbjerg
Carbon Dioxide Activation Center (CADIAC), Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus (Denmark) http://www.skrydstrup-group.com
Search for more papers by this authorCorresponding Author
Prof. Dr. Troels Skrydstrup
Carbon Dioxide Activation Center (CADIAC), Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus (Denmark) http://www.skrydstrup-group.com
Carbon Dioxide Activation Center (CADIAC), Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus (Denmark) http://www.skrydstrup-group.comSearch for more papers by this authorWe are grateful to Dr. Jacob Overgaard for the X-ray crystallographic analysis. We are deeply appreciative of generous financial support from the Danish National Research Foundation, (grant no. DNRF118), the Villum Foundation, the Danish Council for Independent Research: Technology and Production Sciences, the Lundbeck Foundation, the Carlsberg Foundation, and Aarhus University. We also thank the Chinese Scholarship Council for a grant to Z.X.
Graphical Abstract
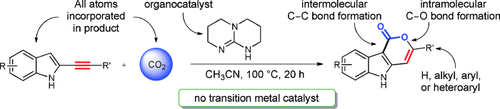
Caught in a trap: The first trapping of CO2 through organocatalyzed CC and CO bond formation is reported. By using alkynyl indoles, this method generates novel indole lactone derivatives by using as little as 5 mol % of the simple organic base 1,5,7-triazabicyclo-[4.4.0]dec-5-ene as an organocatalyst. The transformation shows excellent atom economy and a broad substrate scope, including aromatic, heteroaromatic, and aliphatic 2-alkynyl indoles.
Abstract
The first organocatalyzed trapping of CO2 through CC and CO bond formation is reported. Alkynyl indoles together with catalytic amounts of an organic base and five equivalents of CO2 resulted in the formation new heterocyclic structures. These tricyclic indole-containing products were successfully prepared under mild reaction conditions from aromatic, heteroaromatic, and aliphatic alkynyl indoles with complete regioselectivity. Further investigations suggest that CC bond formation is the initial intermolecular step, followed by lactone-forming CO bond formation.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201500233_sm_miscellaneous_information.pdf1.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. Zhou, V. Sofiyev, H. Kaur, B. Snyder, M. K. Mankowski, P. Hogan, R. G. Ptak, M. Gochin, J. Med. Chem. 2014, 57, 5270–5281;
- 1bG. Xia, X. Han, X. Lu, Org. Lett. 2014, 16, 2058–2061;
- 1cS. Roy, R. Gajbhiye, M. Mandal, C. Pal, A. Meyyapan, J. Mukherjee, P. Jaisankar, Med. Chem. Res. 2013, 23, 1371–1377;
- 1dN. K. Kaushik, N. Kaushik, P. Attri, N. Kumar, C. H. Kim, A. K. Verma, E. H. Choi, Molecules 2013, 18, 6620–6662;
- 1eS. Biswal, U. Sahoo, S. Sethy, H. K. S. Kumar, M. Banerjee, J. Hooker, Asian J. Pharm. Clin. Res. 2012, 5, 1–6;
- 1fR. Dhani, A. Avinash, S. K. Salenaagina, M. V. S. Teja, P. Masthanaiah, J. Chem. Pharm. Res. 2011, 3, 519–523;
- 1gN. K. Garg, R. Sarpong, B. M. Stoltz, J. Am. Chem. Soc. 2002, 124, 13179–13184.
- 2
- 2aS. G. Subramaniapillai, A. Ganesan, Tetrahedron Lett. 2014, 55, 694–698;
- 2bF.-L. Yang, S.-K. Tian, Angew. Chem. Int. Ed. 2013, 52, 4929–4932; Angew. Chem. 2013, 125, 5029–5032;
- 2cC. D. Prasad, S. Kumar, M. Sattar, A. Adhikary, S. Kumar, Org. Biomol. Chem. 2013, 11, 8036–8040;
- 2dR. Y. Nimje, M. V. Leskinen, P. M. Pihko, Angew. Chem. Int. Ed. 2013, 52, 4818–4822; Angew. Chem. 2013, 125, 4918–4922;
- 2eY. Zhang, D. Stephens, G. Hernandez, R. Mendoza, O. V. Larionov, Chem. Eur. J. 2012, 18, 16612–16615;
- 2fD. Huang, J. Chen, W. Dan, J. Ding, M. Liu, H. Wu, Adv. Synth. Catal. 2012, 354, 2123–2128;
- 2gK. Higuchi, M. Tayu, T. Kawasaki, Chem. Commun. 2011, 47, 6728–6730;
- 2hL. Jiao, T. Bach, J. Am. Chem. Soc. 2011, 133, 12990–12993;
- 2iG. Bartoli, G. Bencivenni, R. Dalpozzo, Chem. Soc. Rev. 2010, 39, 4449–4465;
- 2jS. Beaumont, V. Pons, P. Retailleau, R. H. Dodd, P. Dauban, Angew. Chem. Int. Ed. 2010, 49, 1634–1637; Angew. Chem. 2010, 122, 1678–1681;
- 2kI. A. Sayyed, K. Alex, A. Tillack, N. Schwarz, A. Spannenberg, D. Michalik, M. Beller, Tetrahedron 2008, 64, 4590–4595;
- 2lA. Maehara, H. Tsurugi, T. Satoh, M. Miura, Org. Lett. 2008, 10, 1159–1162.
- 3For selected reviews on utilization of CO2 as a C1 building block see:
- 3aM. Aresta, A. Dibenedetto, A. Angelini, Chem. Rev. 2014, 114, 1709–1742;
- 3bX. Cai, B. Xie, Synthesis 2013, 3305–3324;
- 3cA. A. Olajire, J. CO2 Util. 2013, 3–4, 74–92;
- 3dY. Tsuji, T. Fujihara, Chem. Commun. 2012, 48, 9956–9964;
- 3eM. Cokoja, C. Bruckmeier, B. Rieger, W. A. Herrmann, F. E. Kühn, Angew. Chem. Int. Ed. 2011, 50, 8510–8537; Angew. Chem. 2011, 123, 8662–8690;
- 3fR. Martín, A. W. Kleij, ChemSusChem 2011, 4, 1259–1263.
- 4
- 4aC. Lescot, D. U. Nielsen, I. S. Makarov, A. T. Lindhardt, K. Daasbjerg, T. Skrydstrup, J. Am. Chem. Soc. 2014, 136, 6142–6147;
- 4bT. Moragas, J. Cornella, R. Martin, J. Am. Chem. Soc. 2014, 136, 17702–17705;
- 4cY. Zhao, B. Yu, Z. Yang, H. Zhang, L. Hao, X. Gao, Z. Liu, Angew. Chem. Int. Ed. 2014, 53, 5922–5925; Angew. Chem. 2014, 126, 6032–6035;
- 4dL. Wu, Q. Liu, I. Fleischer, R. Jackstell, M. Beller, Nat. Commun. 2014, 5, 3091;
- 4eE. Blondiaux, J. Pouessel, T. Cantat, Angew. Chem. Int. Ed. 2014, 53, 12186–12190; Angew. Chem. 2014, 126, 12382–12386;
- 4fA. Tlili, X. Frogneux, E. Blondiaux, T. Cantat, Angew. Chem. Int. Ed. 2014, 53, 2543–2545; Angew. Chem. 2014, 126, 2577–2579;
- 4gD. B. Nale, S. Rana, K. Parida, B. M. Bhanage, Appl. Catal. A 2014, 469, 340–349;
- 4hY. Li, I. Sorribes, T. Yan, K. Junge, M. Beller, Angew. Chem. Int. Ed. 2013, 52, 12156–12160; Angew. Chem. 2013, 125, 12378–12382;
- 4iO. Jacquet, X. Frogneux, C. Das Neves Gomes, T. Cantat, Chem. Sci. 2013, 4, 2127–2131;
- 4jJ. Seayad, A. M. Seayad, J. K. P. Ng, C. L. L. Chai, ChemCatChem 2012, 4, 774–777;
- 4kC. Das Neves Gomes, O. Jacquet, C. Villiers, P. Thuéry, M. Ephritikhine, T. Cantat, Angew. Chem. Int. Ed. 2012, 51, 187–190; Angew. Chem. 2012, 124, 191–194.
- 5K. Nemoto, S. Onozawa, N. Egusa, N. Morohashi, T. Hattori, Tetrahedron Lett. 2009, 50, 4512–4514.
- 6W.-J. Yoo, M. Guiteras Capdevila, X. Du, S. Kobayashi, Org. Lett. 2012, 14, 5326–5329.
- 7For CH methylation of indole using CO2 and H2, see: Y. Li, T. Yan, K. Junge, M. Beller, Angew. Chem. Int. Ed. 2014, 53, 10476–10480; Angew. Chem. 2014, 126, 10644–10648.
- 8
- 8aR. Nicholls, S. Kaufhold, B. N. Nguyen, Catal. Sci. Technol. 2014, 4, 3458–3462;
- 8bS. Hase, Y. Kayaki, T. Ikariya, Organometallics 2013, 32, 5285–5288;
- 8cY. Takeda, S. Okumura, S. Tone, I. Sasaki, S. Minakata, Org. Lett. 2012, 14, 4874–4877;
- 8dY. Kayaki, M. Yamamoto, T. Ikariya, Angew. Chem. Int. Ed. 2009, 48, 4194–4197; Angew. Chem. 2009, 121, 4258–4261.
- 9R. Sundberg in Electrophilic Substitution Reactions of Indoles, Vol. 26 (Ed.: ), Springer, Berlin, 2010, pp. 47–115.
- 10
- 10aT. Wang, S. Shi, M. Rudolph, A. S. K. Hashmi, Adv. Synth. Catal. 2014, 356, 2337–2342;
- 10bM. C. Blanco Jaimes, F. Rominger, M. M. Pereira, R. M. B. Carrilho, S. A. C. Carabineiro, A. S. K. Hashmi, Chem. Commun. 2014, 50, 4937–4940;
- 10cZ. Xin, S. Kramer, J. Overgaard, T. Skrydstrup, Chem. Eur. J. 2014, 20, 7926–7930;
- 10dW. Zi, F. D. Toste, J. Am. Chem. Soc. 2013, 135, 12600–12603;
- 10eS. Kramer, T. Skrydstrup, Angew. Chem. Int. Ed. 2012, 51, 4681–4684; Angew. Chem. 2012, 124, 4759–4762;
- 10fM. Alfonsi, A. Arcadi, M. Aschi, G. Bianchi, F. Marinelli, J. Org. Chem. 2005, 70, 2265–2273.
- 11Results not shown. See the Supporting Information.
- 12
- 12aY. N. Lim, C. Lee, H.-Y. Jang, Eur. J. Org. Chem. 2014, 1823–1826;
- 12bX. Wang, Y. N. Lim, C. Lee, H.-Y. Jang, B. Y. Lee, Eur. J. Org. Chem. 2013, 1867–1871;
- 12cX. Fu, C.-H. Tan, Chem. Commun. 2011, 47, 8210–8222;
- 12dC. Villiers, J.-P. Dognon, R. Pollet, P. Thuéry, M. Ephritikhine, Angew. Chem. Int. Ed. 2010, 49, 3465–3468; Angew. Chem. 2010, 122, 3543–3546;
- 12eF. S. Pereira, E. R. de Azevedo, E. F. da Silva, T. J. Bonagamba, D. L. da Silva Agostíni, A. Magalhães, A. E. Job, E. R. P. González, Tetrahedron 2008, 64, 10097–10106;
- 12fX. Zhang, N. Zhao, W. Wei, Y. Sun, Catal. Today 2006, 115, 102–106.
- 13Two additional substrates 2-(phenylethynyl)benzo[b]thiophene and N-methyl-3-(phenylethynyl)aniline were examined.
- 14A m/z of 262.0875 corresponding to 3-carboxylation of the indole, could be observed in HRMS in negative mode. Attempts to isolate and characterize this product were unsuccessful.