Palladium-Catalyzed Enantioselective CH Arylation for the Synthesis of P-Stereogenic Compounds†
Zi-Qi Lin
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)
Search for more papers by this authorWei-Zhen Wang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)
Search for more papers by this authorShao-Bai Yan
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Wei-Liang Duan
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)Search for more papers by this authorZi-Qi Lin
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)
Search for more papers by this authorWei-Zhen Wang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)
Search for more papers by this authorShao-Bai Yan
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Wei-Liang Duan
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)Search for more papers by this authorThis work was financially supported by the National Basic Research Program of China (973 Program 2010CB833300) and NSFC (20902099, 21172238, and 21472218).
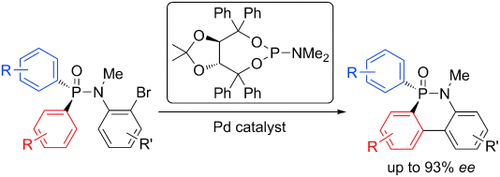
Graphical Abstract
Dressing up phosphorus: The title reaction of N-(o-bromoaryl)-diarylphosphinic amides is described for the synthesis of phosphorus compounds bearing a P-stereogenic center. The method provides good enantioselectivities and high yields. The products were readily transformed into P-chiral biphenyl monophosphine ligands.
Abstract
A palladium-catalyzed enantioselective CH arylation of N-(o-bromoaryl)-diarylphosphinic amides is described for the synthesis of phosphorus compounds bearing a P-stereogenic center. The method provides good enantioselectivities and high yields. The products were readily transformed into P-chiral biphenyl monophosphine ligands.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201500201_sm_miscellaneous_information.pdf1.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Phosphorus Heterocycles II (Topics in Heterocyclic Chemistry Vol. 21), (Eds.: R. K. Bansal), Springer, Berlin, 2010;
- 1b Phosphorus Ligands in Asymmetric Catalysis: Synthesis and Applications, Vols. 1–3 (Eds.: ), Wiley-VCH, Weinheim, 2008;
- 1c Phosphorus Compounds: Advanced Tools in Catalysis and Material Sciences (Eds: ), Springer, Berlin, 2011.
- 2For reviews on the synthesis of P-chiral phosphines, see:
- 2aK. M. Pietrusiewicz, M. Zablocka, Chem. Rev. 1994, 94, 1375;
- 2bA. Grabulosa, J. Granell, G. Muller, Coord. Chem. Rev. 2007, 251, 25;
- 2cO. I. Kolodiazhnyi, Tetrahedron: Asymmetry 2012, 23, 1.
- 3For selected examples of P-chiral ligands in catalysis, see:
- 3aL. Horner, H. Siegel, H. Büthe, Angew. Chem. Int. Ed. Engl. 1968, 7, 942; Angew. Chem. 1968, 80, 1034;
- 3bW. S. Knowles, M. J. Sabacky, Chem. Commun. 1968, 1445;
- 3cW. S. Knowles, M. J. Sabacky, B. D. Vineyard, Chem. Commun. 1972, 10;
- 3dW. S. Knowles, M. J. Sabacky, B. D. Vineyard, D. J. Weinkauff, J. Am. Chem. Soc. 1975, 97, 2567;
- 3eT. Imamoto, J. Watanabe, Y. Wada, H. Masuda, H. Yamada, H. Tsuruta, S. Matsukawa, K. Yamaguchi, J. Am. Chem. Soc. 1998, 120, 1635;
- 3fI. D. Gridnev, N. Higashi, K. Asakura, T. Imamoto, J. Am. Chem. Soc. 2000, 122, 7183;
- 3gW. Tang, X. Zhang, Angew. Chem. Int. Ed. 2002, 41, 1612;
10.1002/1521-3773(20020503)41:9<1612::AID-ANIE1612>3.0.CO;2-H CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 1682;
- 3hW. Tang, W. Wang, X. Zhang, Angew. Chem. Int. Ed. 2003, 42, 943; Angew. Chem. 2003, 115, 973;
- 3iW. Tang, W. Wang, Y. Chi, X. Zhang, Angew. Chem. Int. Ed. 2003, 42, 3509; Angew. Chem. 2003, 115, 3633;
- 3jT. Imamoto, K. Sugita, K. Yoshida, J. Am. Chem. Soc. 2005, 127, 11934;
- 3kT. Imamoto, Y. Saitoh, A. Koide, T. Ogura, K. Yoshida, Angew. Chem. Int. Ed. 2007, 46, 8636; Angew. Chem. 2007, 119, 8790;
- 3lA. M. Taylor, R. A. Altman, S. L. Buchwald, J. Am. Chem. Soc. 2009, 131, 9900;
- 3mT. Imamoto, K. Tamura, Z. Zhang, Y. Horiuchi, M. Sugiya, K. Yoshida, A. Yanagisawa, I. D. Gridnev, J. Am. Chem. Soc. 2012, 134, 1754;
- 3nG. Liu, X. Liu, Z. Cai, G. Jiao, G. Xu, W. Tang, Angew. Chem. Int. Ed. 2013, 52, 4235; Angew. Chem. 2013, 125, 4329.
- 4For reviews on the synthesis of chiral phosphorus compounds, see:
- 4aD. S. Glueck, Synlett 2007, 2627;
- 4bD. S. Glueck, Chem. Eur. J. 2008, 14, 7108;
- 4cJ. S. Harvey, V. Gouverneur, Chem. Commun. 2010, 46, 7477;
- 4dD. Zhao, R. Wang, Chem. Soc. Rev. 2012, 41, 2095.
- 5
- 5aJ. R. Moncarz, N. F. Laritcheva, D. S. Glueck, J. Am. Chem. Soc. 2002, 124, 13356;
- 5bV. S. Chan, I. C. Stewart, R. G. Bergman, F. D. Toste, J. Am. Chem. Soc. 2006, 128, 2786;
- 5cC. Scriban, D. S. Glueck, J. Am. Chem. Soc. 2006, 128, 2788;
- 5dN. F. Blank, J. R. Moncarz, T. J. Brunker, C. Scriban, B. J. Anderson, O. Amir, D. S. Glueck, L. N. Zakharov, J. A. Golen, C. D. Incarvito, A. L. Rheingold, J. Am. Chem. Soc. 2007, 129, 6847;
- 5eV. S. Chan, R. G. Bergman, F. D. Toste, J. Am. Chem. Soc. 2007, 129, 15122;
- 5fC. Scriban, D. S. Glueck, J. A. Golen, A. L. Rheingold, Organometallics 2007, 26, 1788;
- 5gB. J. Anderson, M. A. Guino-o, D. S. Glueck, J. A. Golen, A. G. DiPasquale, L. M. Liable-Sands, A. L. Rheingold, Org. Lett. 2008, 10, 4428;
- 5hV. S. Chan, M. Chiu, R. G. Bergman, F. D. Toste, J. Am. Chem. Soc. 2009, 131, 6021;
- 5iT. W. Chapp, D. S. Glueck, J. A. Golen, C. E. Moore, A. L. Rheingold, Organometallics 2010, 29, 378;
- 5jC. Li, W.-X. Li, S. Xu, W.-L. Duan, Chin. J. Org. Chem. 2013, 33, 799.
- 6
- 6aI. Kovacik, D. K. Wicht, N. S. Grewal, D. S. Glueck, C. D. Incarvito, I. A. Guzei, A. L. Rheingold, Organometallics 2000, 19, 950;
- 6bC. Scriban, I. Kovacik, D. S. Glueck, Organometallics 2005, 24, 4871;
- 6cY. Huang, S. A. Pullarkat, Y. Li, P.-H. Leung, Inorg. Chem. 2012, 51, 2533;
- 6dY. Huang, Y. Li, P.-H. Leung, T. Hayashi, J. Am. Chem. Soc. 2014, 136, 4865;
- 6eC. Li, W.-X. Li, S. Xu, W.-L. Duan, Org. Chem. Front. 2014, 1, 541.
- 7For selected reviews on CH bond functionalization, see:
- 7aF. Kakiuchi, S. Murai, Acc. Chem. Res. 2002, 35, 826;
- 7bF. Kakiuchi, N. Chatani, Adv. Synth. Catal. 2003, 345, 1077;
- 7cB. Li, S. Yang, Z. Shi, Synlett 2008, 949;
- 7dX. Chen, K. M. Engle, D.-H. Wang, J.-Q. Yu, Angew. Chem. Int. Ed. 2009, 48, 5094; Angew. Chem. 2009, 121, 5196;
- 7eR. Giri, B.-F. Shi, K. M. Engle, N. Maugel, J.-Q. Yu, Chem. Soc. Rev. 2009, 38, 3242;
- 7fL. Ackermann, R. Vicente, A. Kapdi, Angew. Chem. Int. Ed. 2009, 48, 9792; Angew. Chem. 2009, 121, 9976;
- 7gL.-M. Xu, B.-J. Li, Z. Yang, Z.-J. Shi, Chem. Soc. Rev. 2010, 39, 712;
- 7hM. Albrecht, Chem. Rev. 2010, 110, 576;
- 7iD. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624;
- 7jT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147;
- 7kL. Ackermann, Chem. Rev. 2011, 111, 1315;
- 7lN. Kuhl, M. N. Hopkinson, J. Wencel-Delord, F. Glorius, Angew. Chem. Int. Ed. 2012, 51, 10236; Angew. Chem. 2012, 124, 10382;
- 7mG. Song, F. Wang, X. Li, Chem. Soc. Rev. 2012, 41, 3651;
- 7nT.-S. Mei, L. Kou, S. Ma, K. M.; Engle, J.-Q. Yu, Synthesis 2012, 1778;
- 7oJ. Yamaguchi, A. D. Yamaguchi, K. Itami, Angew. Chem. Int. Ed. 2012, 51, 8960; Angew. Chem. 2012, 124, 9092;
- 7pJ. Wencel-Delord, F. Glorius, Nat. Chem. 2012, 5, 369;
- 7qN. Yoshikai, Y. Wei, Asian J. Org. Chem. 2013, 2, 466.
- 8For reviews on enantioselective CH activation, see:
- 8aJ. Wencel-Delord, F. Colobert, Chem. Eur. J. 2013, 19, 14010;
- 8bK. M. Engle, J.-Q. Yu, J. Org. Chem. 2013, 78, 8927;
- 8cC. Zheng, S.-L. You, RSC Adv. 2014, 4, 6173.
- 9For selected examples of palladium-catalyzed enantioselective CH activation, see:
- 9aK. Mikami, M. Hatano, M. Terada, Chem. Lett. 1999, 55;
- 9bB.-F. Shi, N. Maugel, Y.-H. Zhang, J.-Q. Yu, Angew. Chem. Int. Ed. 2008, 47, 4882; Angew. Chem. 2008, 120, 4960;
- 9cD. J. Covell, M. C. White, Angew. Chem. Int. Ed. 2008, 47, 6448; Angew. Chem. 2008, 120, 6548;
- 9dM. R. Albicker, N. Cramer, Angew. Chem. Int. Ed. 2009, 48, 9139; Angew. Chem. 2009, 121, 9303;
- 9eB.-F. Shi, Y.-H. Zhang, J. K. Lam, D.-H. Wang, J.-Q. Yu, J. Am. Chem. Soc. 2010, 132, 460;
- 9fA. Renaudat, L. Jean-Gérard, R. Jazzar, C. E. Kefalidis, E. Clot, O. Baudoin, Angew. Chem. Int. Ed. 2010, 49, 7261; Angew. Chem. 2010, 122, 7419;
- 9gK. Takenaka, M. Akita, Y. Tanigaki, S. Takizawa, H. Sasai, Org. Lett. 2011, 13, 3506;
- 9hM. Wasa, K. M. Engle, D. W. Lin, E. J. Yoo, J.-Q. Yu, J. Am. Chem. Soc. 2011, 133, 19598;
- 9iM. Nakanishi, D. Katayev, C. Besnard, E. P. Kündig, Angew. Chem. Int. Ed. 2011, 50, 7438; Angew. Chem. 2011, 123, 7576;
- 9jS. Anas, A. Cordi, H. B. Kagan, Chem. Commun. 2011, 47, 11483;
- 9kD. G. Musaev, A. Kaledin, B.-F. Shi, J.-Q. Yu, J. Am. Chem. Soc. 2012, 134, 1690;
- 9lD. Katayev, M. Nakanishi, T. Bürgi, E. P. Kündig, Chem. Sci. 2012, 3, 1422;
- 9mT. Saget, S. J. Lemouzy, N. Cramer, Angew. Chem. Int. Ed. 2012, 51, 2238; Angew. Chem. 2012, 124, 2281;
- 9nN. Martin, C. Pierre, M. Davi, R. Jazzar, O. Baudoin, Chem. Eur. J. 2012, 18, 4480;
- 9oK. Yamaguchi, J. Yamaguchi, A. Studer, K. Itami, Chem. Sci. 2012, 3, 2165;
- 9pR. Shintani, H. Otomo, K. Ota, T. Hayashi, J. Am. Chem. Soc. 2012, 134, 7305;
- 9qT. Saget, N. Cramer, Angew. Chem. Int. Ed. 2012, 51, 12842; Angew. Chem. 2012, 124, 13014;
- 9rD.-W. Gao, Y.-C. Shi, Q. Gu, Z.-L. Zhao, S.-L. You, J. Am. Chem. Soc. 2013, 135, 86;
- 9sB. M. Trost, D. A. Thaisrivongs, E. J. Donckele, Angew. Chem. Int. Ed. 2013, 52, 1523; Angew. Chem. 2013, 125, 1563;
- 9tT. Saget, N. Cramer, Angew. Chem. Int. Ed. 2013, 52, 7865; Angew. Chem. 2013, 125, 8019;
- 9uD. Katayev, Y.-X. Jia, A. K. Sharma, D. Banerjee, C. Besnard, R. B. Sunoj, E. P. Kündig, Chem. Eur. J. 2013, 19, 11916;
- 9vE. Larionov, M. Nakanishi, D. Katayev, C. Besnard, E. P. Kündig, Chem. Sci. 2013, 4, 1995;
- 9wX.-F. Cheng, Y. Li, Y.-M. Su, F. Yin, J.-Y. Wang, J. Sheng, H. U. Vora, X.-S. Wang, J.-Q. Yu, J. Am. Chem. Soc. 2013, 135, 1236;
- 9xL. Chu, X.-C. Wang, C. E. Moore, A. L. Rheingold, J.-Q. Yu, J. Am. Chem. Soc. 2013, 135, 16344;
- 9yK.-J. Xiao, D. Lin, M. Miura, R.-Y. Zhu, W. Gong, M. Wasa, J.-Q. Yu, J. Am. Chem. Soc. 2014, 136, 8138;
- 9zR. Deng, Y. Huang, X. Ma, G. Li, R. Zhu, B. Wang, Y.-B. Kang, Z. Gu, J. Am. Chem. Soc. 2014, 136, 4472;
- 9aaD.-W. Gao, Q. Yin, Q. Gu, S.-L. You, J. Am. Chem. Soc. 2014, 136, 4841;
- 9abX. Ma, Z. Gu, RSC Adv. 2014, 4, 36241;
- 9acL. Liu, A.-A. Zhang, R.-J. Zhao, F. Li, T.-J. Meng, N. Ishida, M. Murakami, W.-X. Zhao, Org. Lett. 2014, 16, 5336;
- 9adJ. Pedroni, M. Boghi, T. Saget, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 9064; Angew. Chem. 2014, 126, 9210;
- 9aeP. S. Wang, H.-C. Lin, Y.-J. Zhai, Z.-Y. Han, L.-Z. Gong, Angew. Chem. Int. Ed. 2014, 53, 12218; Angew. Chem. 2014, 126, 12414;
- 9afL. Chu, K.-J. Xiao, J.-Q. Yu, Science 2014, 346, 451.
- 10For selected examples of rhodium-catalyzed enantioselective CH activation, see:
- 10aF. Kakiuchi, P. Le Gendre, A. Yamada, H. Ohtaki, S. Murai, Tetrahedron: Asymmetry 2000, 11, 2647;
- 10bR. K. Thalji, J. A. Ellman, R. G. Bergman, J. Am. Chem. Soc. 2004, 126, 7192;
- 10cS. J. O’Malley, K. L. Tan, A. Watzke, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2005, 127, 13496;
- 10dD. N. Tran, N. Cramer, Angew. Chem. Int. Ed. 2010, 49, 8181; Angew. Chem. 2010, 122, 8357;
- 10eQ. Li, Z.-X. Yu, Angew. Chem. Int. Ed. 2011, 50, 2144; Angew. Chem. 2011, 123, 2192;
- 10fT. K. Hyster, L. Knörr, T. R. Ward, T. Rovis, Science 2012, 338, 500;
- 10gB. Ye, N. Cramer, Science 2012, 338, 504;
- 10hY. Kuninobu, K. Yamauchi, N. Tamura, T. Seiki, K. Takai, Angew. Chem. Int. Ed. 2013, 52, 1520; Angew. Chem. 2013, 125, 1560;
- 10iB. Ye, N. Cramer, J. Am. Chem. Soc. 2013, 135, 636;
- 10jD. N. Tran, N. Cramer, Angew. Chem. Int. Ed. 2013, 52, 10630; Angew. Chem. 2013, 125, 10824;
- 10kB. Ye, P. A. Donets, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 507; Angew. Chem. 2014, 126, 517;
- 10lB. Ye, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 7896; Angew. Chem. 2014, 126, 8030.
- 11For selected examples of iridium-catalyzed enantioselective CH activation, see:
- 11aR. Aufdenblatten, S. Diezi, A. Togni, Monatsh. Chem. 2000, 131, 1345;
- 11bK. Tsuchikama, M. Kasagawa, Y.-K. Hashimoto, K. Endo, T. Shibata, J. Organomet. Chem. 2008, 693, 3939;
- 11cS. Pan, K. Endo, T. Shibata, Org. Lett. 2011, 13, 4692;
- 11dC. S. Sevov, J. F. Hartwig, J. Am. Chem. Soc. 2013, 135, 2116;
- 11eT. Nishimura, M. Ebe, Y. Nagamoto, T. Hayashi, Chem. Sci. 2013, 4, 4499.
- 12For selected examples of transition-metal-catalyzed CH activation of organophosphorus compounds, see:
- 12aY. Kuninobu, T. Yoshida, K. Takai, J. Org. Chem. 2011, 76, 7370;
- 12bK. Baba, M. Tobisu, N. Chatani, Angew. Chem. Int. Ed. 2013, 52, 11892; Angew. Chem. 2013, 125, 12108;
- 12cL. Y. Chan, S. Kim, T. Ryu, P. H. Lee, Chem. Commun. 2013, 49, 4682;
- 12dH.-L. Wang, R.-B. Hu, H. Zhang, A.-X. Zhou, S.-D. Yang, Org. Lett. 2013, 15, 5302;
- 12eC.-G. Feng, M. Ye, K.-J. Xiao, S. Li, J.-Q. Yu, J. Am. Chem. Soc. 2013, 135, 9322;
- 12fC. Li, T. Yano, N. Ishida, M. Murakami, Angew. Chem. Int. Ed. 2013, 52, 9801; Angew. Chem. 2013, 125, 9983;
- 12gY. Unoh, Y. Hashimoto, D. Takeda, K. Hirano, T. Satoh, M. Miura, Org. Lett. 2013, 15, 3258;
- 12hT. Ryu, J. Kim, Y. Park, S. Kim, P. H. Lee, Org. Lett. 2013, 15, 3986;
- 12iD. Zhao, C. Nimphius, M. Lindale, F. Glorius, Org. Lett. 2013, 15, 4504;
- 12jD. Gwon, D. Lee, J. Kim, S. Park, S. Chang, Chem. Eur. J. 2014, 20, 12421;
- 12kY.-R. Chen, W.-L. Duan, Synthesis 2014, 1067.
- 13During preparation of the manuscript, an example of palladium-catalyzed enantioselective CH arylation reaction for P-stereogenic compound synthesis was reported by Han et al. See: Z.-J. Du, J. Guan, G.-J. Wu, P. Xu, L.-X. Gao, F.-S. Han, J. Am. Chem. Soc. 2015, 137, 632.
- 14For reviews on biaryl monophosphine ligands, see:
- 14aS. L. Buchwald, C. Mauger, G. Mignani, U. Scholzc, Adv. Synth. Catal. 2006, 348, 243;
- 14bD. S. Surry, S. L. Buchwald, Angew. Chem. Int. Ed. 2008, 47, 6338; Angew. Chem. 2008, 120, 6438;
- 14cR. Martin, S. L. Buchwald, Acc. Chem. Res. 2008, 41, 1461;
- 14dD. S. Surry, S. L. Buchwald, Chem. Sci. 2011, 2, 27.
- 15For examples of synthesis of chiral binaphthyl/aryl monophosphine ligands by using chiral starting materials or by an optical resolution pathway, see:
- 15aY. Uozumi, N. Suzuki, A. Ogiwara, T. Hayashi, Tetrahedron 1994, 50, 4293;
- 15bT. Hamada, S. L. Buchwald, Org. Lett. 2003, 4, 999;
- 15cN. J. Kerrigan, E. C. Dunne, C. Cunningham, P. McArdle, K. Gilligan, D. G. Gilheany, Tetrahedron Lett. 2003, 44, 8461.
- 16
- 16aJ.-J. Feng, X.-F. Chen, M. Shi, W.-L. Duan, J. Am. Chem. Soc. 2010, 132, 5562;
- 16bD. Du, W.-L. Duan, Chem. Commun. 2011, 47, 11101;
- 16cY.-R. Chen, W.-L. Duan, Org. Lett. 2011, 13, 5824;
- 16dJ.-J. Feng, M. Huang, Z.-Q. Lin, W.-L. Duan, Adv. Synth. Catal. 2012, 354, 3122;
- 16eD. Du, Z.-Q. Lin, J.-Z. Lu, C. Li, W.-L. Duan, Asian J. Org. Chem. 2013, 2, 392;
- 16fJ. Lu, J. Ye, W.-L. Duan, Org. Lett. 2013, 15, 5016;
- 16gJ. Lu, J. Ye, W.-L. Duan, Chem. Commun. 2014, 50, 698.
- 17For X-ray crystal data, see the Supporting Information for details.
- 18Two atropisomers exist in equilibrium with each other at ambient temperature (25 °C).
- 19For examples of reduction reactions of chiral phosphine oxides to trivalent phosphines, see Ref. [2] and the following literature:
- 19aT. Imamoto, S.-i. Kikuchi, T. Miura, Y. Wada, Org. Lett. 2001, 3, 87;
- 19bK. V. Rajendran, D. G. Gilheany, Chem. Commun. 2012, 48, 817.