Targeted Histone Peptides: Insights into the Spatial Regulation of the Methyltransferase PRC2 by using a Surrogate of Heterotypic Chromatin†
This research was supported by the U.S. National Institutes of Health (grants R37-GM086868 and R01 GM107047) and the STARR Foundation (grant I6-A614). We would like to acknowledge Dr. Miquel Vila-Perello, Dr. Yael David, and Prof. C. David Allis for helpful discussion.
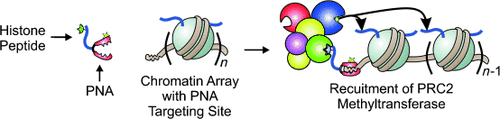
Graphical Abstract
Chromatin PNArrays: A peptide nucleic acid (PNA) targeting compound bearing a modified histone peptide was used to recruit the histone methyltransferase PRC2 to a designated site on a nucleosome array. This recapitulation of heterotypic “designer” chromatin was used to investigate how PRC2 is both activated and inhibited with respect to neighboring nucleosomes from a particular location within the array.
Abstract
Eukaryotic genomes are dynamically regulated through a host of epigenetic stimuli. The substrate for these epigenetic transactions, chromatin, is a polymer of nucleosome building blocks. In native chromatin, each nucleosome can differ from its neighbors as a result of covalent modifications to both the DNA and the histone packaging proteins. The heterotypic nature of chromatin presents a formidable obstacle to biochemical studies seeking to understand the role of context on epigenetic regulation. A chemical approach to the production of heterotypic chromatin that can be used in such studies is introduced. This method involves the attachment of a user-defined modified histone peptide to a designated nucleosome within the polymer by using a peptide nucleic acid (PNA) targeting compound. This strategy was applied to dissect the effect of chromatin context on the activity of the histone methyltransferase PRC2. The results show that PRC2 can be stimulated to produce histone H3 methylation from a defined nucleation site.