Nitrosopurines En Route to Potently Cytotoxic Asmarines†
Kanny K. Wan
Department of Chemistry, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Search for more papers by this authorDr. Kotaro Iwasaki
Department of Chemistry, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Search for more papers by this authorJeffrey C. Umotoy
Department of Molecular and Experimental Medicine and Chemical Physiology, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Dennis W. Wolan
Department of Molecular and Experimental Medicine and Chemical Physiology, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Dennis W. Wolan, Department of Molecular and Experimental Medicine and Chemical Physiology, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Ryan A. Shenvi, Department of Chemistry, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Ryan A. Shenvi
Department of Chemistry, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Dennis W. Wolan, Department of Molecular and Experimental Medicine and Chemical Physiology, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Ryan A. Shenvi, Department of Chemistry, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Search for more papers by this authorKanny K. Wan
Department of Chemistry, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Search for more papers by this authorDr. Kotaro Iwasaki
Department of Chemistry, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Search for more papers by this authorJeffrey C. Umotoy
Department of Molecular and Experimental Medicine and Chemical Physiology, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Dennis W. Wolan
Department of Molecular and Experimental Medicine and Chemical Physiology, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Dennis W. Wolan, Department of Molecular and Experimental Medicine and Chemical Physiology, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Ryan A. Shenvi, Department of Chemistry, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Ryan A. Shenvi
Department of Chemistry, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Dennis W. Wolan, Department of Molecular and Experimental Medicine and Chemical Physiology, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Ryan A. Shenvi, Department of Chemistry, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA)
Search for more papers by this authorThis work was supported by the NIH (GM104180 and the NSF GRFP (K.K.W.; DGE-1346837). We thank Dr. C. E. Moore and Prof. A. L. Rheingold (UCSD) for X-ray crystallographic analysis. We thank TSRI, Eli Lilly, Boehringer Ingelheim, Amgen, the Baxter Foundation, and the Sloan Foundation for additional financial support.
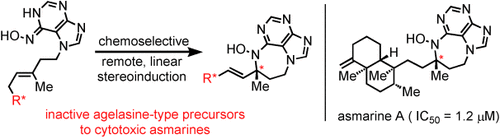
Graphical Abstract
Unnatural product: A nitrosopurine ene reaction easily assembles the asmarine pharmacophore and transmits remote stereochemistry to the diazepine-purine heterocycle. This reaction generates potent cytotoxins which exceed the potency of asmarine A and supersede the metabolites as useful leads for biological discovery.
Abstract
A nitrosopurine ene reaction easily assembles the asmarine pharmacophore and transmits remote stereochemistry to the diazepine-purine hetereocycle. This reaction generates potent cytotoxins which exceed the potency of asmarine A (1.2 μM IC50) and supersede the metabolites as useful leads for biological discovery.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201411493_sm_miscellaneous_information.pdf11.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Yosief, A. Rudi, Z. Stein, I. Goldberg, G. M. D. Gravalos, M. Schleyer, Y. Kashman, Tetrahedron Lett. 1998, 39, 3323–3326;
- 1bT. Yosief, A. Rudi, Y. Kashman, J. Nat. Prod. 2000, 63, 299–304.
- 2D. Pappo, S. Shimony, Y. Kashman, J. Org. Chem. 2005, 70, 199–206.
- 3
- 3aH. Nakamura, H. Wu, Y. Ohizumi, Y. Hirata, Tetrahedron Lett. 1984, 25, 2989;
- 3bA. A. Pimentel, P. Felibertt, F. Sojo, L. Colman, A. Mayora, M. L. Silva, H. Rojas, R. Dipolo, A. I. Suarez, R. S. Compagnone, F. Arvelo, I. Galindo-Castro, J. B. De Sanctis, P. Chirino, G. Benaim, Cancer Chemother. Pharmacol. 2012, 69, 71;
- 3cR. Fathi-Afshar, T. M. Allen, C. A. Krueger, D. A. Cook, A. S. Clanachan, R. Vriend, H. P. Baer, C. E. Cass, Can. J. Physiol. Pharmacol. 1989, 67, 276.
- 4A. Vik, E. Hedner, C. Charnock, L. W. Tangen, Ø Samuelsen, R. Larsson, L. Bohlin, L.-L. Gundersen, Bioorg. Med. Chem. 2007, 15, 4016; Samuelsen, R. Larsson, L. Bohlin, L.-L. Gundersen, Bioorg. Med. Chem. 2007, 15, 4016.
- 5Y. Kashman, personal communication.
- 6
- 6aD. Pappo, A. Rudi, Y. Kashman, Tetrahedron Lett. 2001, 42, 5941;
- 6bM. Ohba, T. Tashiro, Heterocycles 2002, 57, 1235;
- 6cS. A. Rodgen, S. E. Schaus, Angew. Chem. Int. Ed. 2006, 45, 4929; Angew. Chem. 2006, 118, 5051;
- 6dA. Vik, L.-L. Gundersen, Tetrahedron Lett. 2007, 48, 1931.
- 7Although labeled as cytotoxicity in Ref. [2], the use of GI50 indicates cytostatic effect. Based on the activity of our analogues, we assume the authors observed cytotoxicity and meant to use EC50.
- 8W. Adam, O. Krebs, Chem. Rev. 2003, 103, 4131.
- 9
- 9aG. E. Keck, R. R. Webb, J. Org. Chem. 1982, 47, 1302;
- 9bW. Adam, H.-G. Degen, O. Krebs, C. R. Saha-Möller, J. Am. Chem. Soc. 2002, 124, 12938;
- 9cW. Oppolzer, G. Poli, C. Starkemann, G. Bernardinelli, Tetrahedron Lett. 1988, 29, 3559;
- 9dG. Kreße, A. Vasella, H. Felber, A. Ritter, B. Ascherl, Recl. Trav. Chim. Pays-Bas 1986, 105, 295;
- 9eW. Adam, N. Bottke, J. Am. Chem. Soc. 2000, 122, 9846;
- 9fW. Adam, N. Bottke, O. Krebs, I. Lykakis, M. Orfanopoulos, M. Stratakis, J. Am. Chem. Soc. 2002, 124, 14403.
- 10G. E. Keck, R. R. Webb, J. B. Yates, Tetrahedron Lett. 1981, 37, 4007.
- 11P. Wipf, S. Lim, Angew. Chem. Int. Ed. Engl. 1993, 32, 1068; Angew. Chem. 1993, 105, 1095.
- 12J. Chen, S. Ma, J. Org. Chem. 2009, 74, 5595.
- 13N. Okukado, E. Negishi, Tetrahedron Lett. 1978, 19, 2357.
10.1016/S0040-4039(01)94773-2 Google Scholar
- 14J. A. Montgomery, K. Hewson, J. Org. Chem. 1961, 26, 4469.
- 15T. Tokoroyama, Synthesis 2000, 611.
- 16T. J. Donohoe, P. M. Guyo, M. Helliwell, Tetrahedron Lett. 1999, 40, 435.
- 17R. O. Duthaler, J. D. Roberts, J. Am. Chem. Soc. 1978, 100, 4969.
- 18A. Giner-Sorolla, J. Heterocycl. Chem. 1970, 7, 75.
- 19A. G. Leach, K. N. Houk, J. Am. Chem. Soc. 2002, 124, 14820.
- 20CCDC 1036462 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 21L.-L. Gundersen, Phytochem. Rev. 2013, 12, 467.
- 22E.-I. Negishi, Q. Hu, Z. Huang, M. Qian, G. Wang, Aldrichimica Acta 2005, 38, 2005.
- 23CCDC 1036478 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.