Ratiometric Fluorescence Imaging of Cellular Polarity: Decrease in Mitochondrial Polarity in Cancer Cells†
Na Jiang
State Key Laboratory of Fine Chemicals, Dalian University of Technology, 2 Linggong Road, 116024 Dalian (China)
Search for more papers by this authorCorresponding Author
Jiangli Fan
State Key Laboratory of Fine Chemicals, Dalian University of Technology, 2 Linggong Road, 116024 Dalian (China)
State Key Laboratory of Fine Chemicals, Dalian University of Technology, 2 Linggong Road, 116024 Dalian (China)Search for more papers by this authorFeng Xu
State Key Laboratory of Fine Chemicals, Dalian University of Technology, 2 Linggong Road, 116024 Dalian (China)
Search for more papers by this authorProf. Xiaojun Peng
State Key Laboratory of Fine Chemicals, Dalian University of Technology, 2 Linggong Road, 116024 Dalian (China)
Search for more papers by this authorHuiying Mu
State Key Laboratory of Fine Chemicals, Dalian University of Technology, 2 Linggong Road, 116024 Dalian (China)
Search for more papers by this authorProf. Jingyun Wang
School of Life Science and Biotechnology, Dalian University of Technology, 2 Linggong Road, 116024 Dalian (China)
Search for more papers by this authorXiaoqing Xiong
State Key Laboratory of Fine Chemicals, Dalian University of Technology, 2 Linggong Road, 116024 Dalian (China)
Search for more papers by this authorNa Jiang
State Key Laboratory of Fine Chemicals, Dalian University of Technology, 2 Linggong Road, 116024 Dalian (China)
Search for more papers by this authorCorresponding Author
Jiangli Fan
State Key Laboratory of Fine Chemicals, Dalian University of Technology, 2 Linggong Road, 116024 Dalian (China)
State Key Laboratory of Fine Chemicals, Dalian University of Technology, 2 Linggong Road, 116024 Dalian (China)Search for more papers by this authorFeng Xu
State Key Laboratory of Fine Chemicals, Dalian University of Technology, 2 Linggong Road, 116024 Dalian (China)
Search for more papers by this authorProf. Xiaojun Peng
State Key Laboratory of Fine Chemicals, Dalian University of Technology, 2 Linggong Road, 116024 Dalian (China)
Search for more papers by this authorHuiying Mu
State Key Laboratory of Fine Chemicals, Dalian University of Technology, 2 Linggong Road, 116024 Dalian (China)
Search for more papers by this authorProf. Jingyun Wang
School of Life Science and Biotechnology, Dalian University of Technology, 2 Linggong Road, 116024 Dalian (China)
Search for more papers by this authorXiaoqing Xiong
State Key Laboratory of Fine Chemicals, Dalian University of Technology, 2 Linggong Road, 116024 Dalian (China)
Search for more papers by this authorThis research was financially supported by the NSF of China (21136002, 21376039, 21422601, and 21421005), the National Basic Research Program of China (2013CB733702), the Ministry of Education (NCET-12-0080), Liaoning NSF (2013020115), and Fundamental Research Funds for the Central Universities (DUT14ZD214).
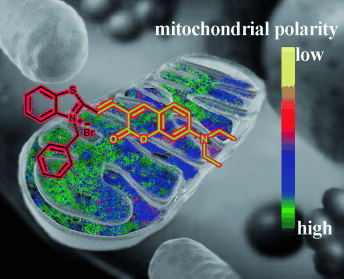
Graphical Abstract
…︁ and BOB's your uncle: A fluorescent probe of mitochondrial polarity, termed BOB, showed a linear ratiometric fluorescence response to solution polarity. Various mitochondria of normal cells and cancer cells were examined, and it was found that mitochondrial polarity tends to be lower in cancer cells than in normal cells. The detection of mitochondrial polarity could thus be used as a method to distinguish cancer cells from normal cells.
Abstract
Mitochondrial polarity strongly influences the intracellular transportation of proteins and interactions between biomacromolecules. The first fluorescent probe capable of the ratiometric imaging of mitochondrial polarity is reported. The probe, termed BOB, has two absorption maxima (λabs=426 and 561 nm) and two emission maxima—a strong green emission (λem=467 nm) and a weak red emission (642 nm in methanol)—when excited at 405 nm. However, only the green emission is markedly sensitive to polarity changes, thus providing a ratiometric fluorescence response with a good linear relationship in both extensive and narrow ranges of solution polarity. BOB possesses high specificity to mitochondria (Rr=0.96) that is independent of the mitochondrial membrane potential. The mitochondrial polarity in cancer cells was found to be lower than that of normal cells by ratiometric fluorescence imaging with BOB. The difference in mitochondrial polarity might be used to distinguish cancer cells from normal cells.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201410645_sm_miscellaneous_information.pdf1.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. Ercelen, A. S. Klymchenko, A. P. Demchenko, Anal. Chim. Acta 2002, 464, 273–287.
- 2Z. G. Yang, J. F. Cao, Y. X. He, J. H. Yang, T. Kim, X. J. Peng, J. S. Kim, Chem. Soc. Rev. 2014, 43, 4563–4601.
- 3
- 3aY. D. Zhuang, P. Y. Chiang, C. W. Wang, K. T. Tan, Angew. Chem. Int. Ed. 2013, 52, 8124–8128; Angew. Chem. 2013, 125, 8282–8286;
- 3bM. W. Berns, T. Krasieva, C. H. Sun, A. Dvornikov, P. M. Rentzepis, J. Photochem. Photobiol. B 2004, 19, 51–56;
- 3cL. Huang, S. W. Tam-Chang, J. Fluoresc. 2011, 21, 213–222.
- 4
- 4aB. Szczupak, A. G. Ryder, D. M. Togashi, A. S. Klymchenko, Y. A. Rochev, A. Gorelov, T. J. Glynn, J. Fluoresc. 2010, 20, 719–731;
- 4bP. Suppan, J. Chem. Soc. A 1968, 3125–3133.
- 5
- 5aL. Wang, Y. Xiao, W. M. Tian, L. Z. Deng, J. Am. Chem. Soc. 2013, 135, 2903–2906;
- 5bM. K. Kuimova, S. W. Botchway, A. W. Parker, M. Balaz, H. A. Collins, H. L. Anderson, K. Suhling, P. R. Ogilby, Nat. Chem. 2009, 1, 69–73;
- 5cY. Koide, Y. Urano, S. Kenmoku, H. Kojima, T. Nagano, J. Am. Chem. Soc. 2007, 129, 10324–10325;
- 5dS. Chalmers, S. T. Caldwell, C. Quin, T. A. Prime, A. M. James, A. G. Cairns, M. P. Murphy, J. G. McCarron, R. C. Hartley, J. Am. Chem. Soc. 2012, 134, 758–761.
- 6
- 6aH. Rottenberg, Biochemistry 1992, 31, 9473–9481;
- 6bG. L. Martin, C. Lau, S. D. Minteer, M. J. Cooney, Analyst 2010, 135, 1131–1137;
- 6cC. W. T. Leung, Y. Hong, S. Chen, E. Zhao, J. W. Y. Lam, B. Z. Tang, J. Am. Chem. Soc. 2013, 135, 4926–4929.
- 7
- 7aP. H. Reddy, M. F. Beal, Brain Res. Rev. 2005, 49, 618–632;
- 7bH. M. McBride, M. Neuspiel, S. Wasiak, Curr. Biol. 2006, 16, 551–560;
- 7cJ. Estaquier, D. Arnoult, Cell Death Differ. 2007, 14, 1086–1094.
- 8
- 8aD. Wang, J. Wang, G. M. C. Bonamy, S. Meeusen, R. G. Brusch, C. Turk, P. Yang, P. G. Schultz, Angew. Chem. Int. Ed. 2012, 51, 9302–9305; Angew. Chem. 2012, 124, 9436–9439;
- 8bT. Landes, J. C. Martinou, Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 540–545;
- 8cL. F. Yousif, K. M. Stewart, S. O. Kelley, ChemBioChem 2009, 10, 1939–1950.
- 9
- 9aR. A. Musrati, M. Kollarova, N. Mernik, D. Mikulasova, Gen. Physiol. Biophys. 1998, 17, 193–210;
- 9bJ. W. Callahan, G. W. Kosicki, Can. J. Biochem. 1967, 45, 839–850.
- 10Y. Zhou, J. F. Zhang, J. Yoon, Chem. Rev. 2014, 114, 5511–5571.
- 11
- 11aG. Saroja, T. Soujanya, B. Ramachandram, A. Samanta, J. Fluoresc. 1998, 8, 405–410;
- 11bH. Sauer, L. Pratsch, R. Peters, Anal. Biochem. 1991, 194, 418–424;
- 11cK. Kudo, A. Momotake, J. K. Tanaka, Y. Miwa, T. Arai, Photochem. Photobiol. Sci. 2012, 11, 674–678;
- 11dH. Sunahara, Y. Urano, H. Kojima, T. Nagano, J. Am. Chem. Soc. 2007, 129, 5597–5604;
- 11eO. A. Kucherak, S. Oncul, Z. Darwich, D. A. Yushchenko, Y. Arntz, P. Didier, Y. Mély, A. S. Klymchenko, J. Am. Chem. Soc. 2010, 132, 4907–4916;
- 11fG. Loving, B. Imperiali, J. Am. Chem. Soc. 2008, 130, 13630–13638;
- 11gC. Huang, Q. Yin, W. Zhu, Y. Yang, X. Wang, X. Qian, Y. Xu, Angew. Chem. Int. Ed. 2011, 50, 7551–7556; Angew. Chem. 2011, 123, 7693–7698;
- 11hF. Amin, D. A. Yushchenko, J. M. Montenegro, W. J. Parak, ChemPhysChem 2012, 13, 1030–1035.
- 12aA. Toutchkine, V. Kraynov, K. Hahn, J. Am. Chem. Soc. 2003, 125, 4132–4145;
- 12bG. Signore, R. Nifosì, L. Albertazzi, B. Storti, R. Bizzarri, J. Am. Chem. Soc. 2010, 132, 1276–1288.
- 13
- 13aJ. Massin, A. Charaf-Eddin, F. Appaix, Y. Bretonnière, D. Jacquemin, B. Sanden, C. Monnereau, C. Andraud, Chem. Sci. 2013, 4, 2833–2843;
- 13bS. Giovanni, N. Riccardo, A. Lorenzo, B. Ranieri, J. Biomed. Nanotechnol. 2009, 5, 722–729;
- 13cM. Koenig, G. Bottari, G. Brancato, V. Barone, D. M. Guldi, T. Torres, Chem. Sci. 2013, 4, 2502–2511.
- 14
- 14aH. Turki, S. Abid, S. Fery-Forgues, R. E. Gharbi, Dyes Pigm. 2007, 73, 311–316;
- 14bT. Hirano, K. Hiromoto, H. Kagechika, Org. Lett. 2007, 9, 1315–1318.
- 15
- 15a“One-class bi-benzyl pentamethyl cyanine fluorescent dye, and preparation method and application thereof”: J. Fan, L. A. Lee, X. Peng, X. Qiang, Q. Wang, China Patent. WO2013075285A1, 2013;
- 15bM. H. Lee, N. Park, C. Yi, J. H. Han, J. H. Hong, K. P. Kim, D. H. Kang, J. L. Sessler, C. Kang, J. S. Kim, J. Am. Chem. Soc. 2014, 136, 8430–8437.
- 16
- 16aW. Qin, M. Baruah, M. Sliwa, M. V. D. Auweraer, W. M. D. Borggraeve, D. Beljonne, B. V. Averbeke, N. Boens, J. Phys. Chem. A 2008, 112, 6104–6114;
- 16bA. Niemann, A. Takatsuki, H. P. Elsässer, J. Histochem. Cytochem. 2000, 48, 251–258;
- 16cR. C. Weast, Handbook of Chemistry and Physics, CRC, Boca Raton, FL, 1980.
- 17J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, 3rd ed., 2010.
- 18
- 18aL. Ding, Z. Zhang, X. Li, J. Su, Chem. Commun. 2013, 49, 7319–7321;
- 18bL. Yang, X. Li, J. B. Yang, Y. Qu, J. L. Hua, ACS Appl. Mater. Interfaces 2013, 5, 1317–1326.
- 19J. S. Lee, Y. K. Kim, M. Vendrell, Y. T. Chang, Mol. BioSyst. 2009, 5, 411–421.
- 20
- 20aF. Rashid, R. W. Horobin, Histochemistry. 1990, 94, 303–308;
- 20bR. W. Horobin, J. C. Stockert, F. Rashid-Doubell, Histochem. Cell Biol. 2006, 126, 165–175.
- 21
- 21aM. H. Lee, J. H. Han, J. H. Lee, H. G. Choi, C. Kang, J. S. Kim, J. Am. Chem. Soc. 2012, 134, 17314–17319;
- 21bK. Sreenath, J. R. Allen, M. W. Davidson, L. Zhu, Chem. Commun. 2011, 47, 11730–11732.
- 22
- 22aA. Harriman, L. J. Mallon, G. Ulrich, R. Ziessel, ChemPhysChem 2007, 8, 1207–1214;
- 22bC. S. Russell, Jr., W. G. Lee, Biophys. J. 1999, 76, 469–477.
- 23H. Kobayashi, M. Ogawa, R. Alford, P. L. Choyke, Y. Urano, Chem. Rev. 2010, 110, 2620–2640.
- 24Q. Y. Chen, X. Lv, Z. J. Zhang, H. Dai, J. Clin. Exp. Med. 2011, 10, 347–348.