One-Pot Transformation of Simple Furans into 4-Hydroxy-2-cyclopentenones in Water†
Dr. Dimitris Kalaitzakis
Department of Chemistry, University of Crete, Vasilika Vouton, 71003, Iraklion, Crete (Greece)
Search for more papers by this authorMyron Triantafyllakis
Department of Chemistry, University of Crete, Vasilika Vouton, 71003, Iraklion, Crete (Greece)
Search for more papers by this authorIoanna Alexopoulou
Department of Chemistry, University of Crete, Vasilika Vouton, 71003, Iraklion, Crete (Greece)
Search for more papers by this authorManolis Sofiadis
Department of Chemistry, University of Crete, Vasilika Vouton, 71003, Iraklion, Crete (Greece)
Search for more papers by this authorCorresponding Author
Prof. Dr. Georgios Vassilikogiannakis
Department of Chemistry, University of Crete, Vasilika Vouton, 71003, Iraklion, Crete (Greece)
Department of Chemistry, University of Crete, Vasilika Vouton, 71003, Iraklion, Crete (Greece)Search for more papers by this authorDr. Dimitris Kalaitzakis
Department of Chemistry, University of Crete, Vasilika Vouton, 71003, Iraklion, Crete (Greece)
Search for more papers by this authorMyron Triantafyllakis
Department of Chemistry, University of Crete, Vasilika Vouton, 71003, Iraklion, Crete (Greece)
Search for more papers by this authorIoanna Alexopoulou
Department of Chemistry, University of Crete, Vasilika Vouton, 71003, Iraklion, Crete (Greece)
Search for more papers by this authorManolis Sofiadis
Department of Chemistry, University of Crete, Vasilika Vouton, 71003, Iraklion, Crete (Greece)
Search for more papers by this authorCorresponding Author
Prof. Dr. Georgios Vassilikogiannakis
Department of Chemistry, University of Crete, Vasilika Vouton, 71003, Iraklion, Crete (Greece)
Department of Chemistry, University of Crete, Vasilika Vouton, 71003, Iraklion, Crete (Greece)Search for more papers by this authorThe research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement number 277588. The authors also wish to acknowledge co-funding by the European Regional Development Fund of the EU and national funds—Greek Ministry of Education and Religious Affairs, Sport and Culture/GGET—EYDE-ETAK, through the Operational Program Competitiveness and Entrepreneurship (OPC II), NSRF 2007-2013, Action “SYNERGASIA 2011” Project: THERA-CAN—number 11ΣYN_1_485. This research has been co-financed by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program: Heracleitus II (Ph.D. fellowship for I.A.). Investing in knowledge society through the European Social Fund.
Graphical Abstract
Abstract
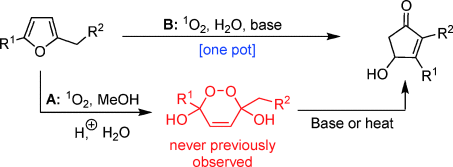
A highly efficient one-pot transformation of readily accessible furans into 4-hydroxy-2-cyclopentenones in H2O, using singlet oxygen as oxidant, has been developed.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201407477_sm_miscellaneous_information.pdf7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected recent examples of natural products containing the scaffold, see:
- 1aS. Greff, M. Zubia, G. Genta-Jouve, L. Massi, T. Perez, O. P. Thomas, J. Nat. Prod. 2014, 77, 1150–1155;
- 1bJ. Urban, C. J. Dahlberg, B. J. Carroll, W. Kaminsky, Angew. Chem. Int. Ed. 2013, 52, 1553–1555; Angew. Chem. 2013, 125, 1593–1595;
- 1cV. Schmidts, M. Fredersdorf, T. Lübken, A. Porzel, N. Arnold, L. Wessjohann, C. M. Thiele, J. Nat. Prod. 2013, 76, 839–844;
- 1dS. Wang, S.-S. Liu, Z.-M. Lin, R.-J. Li, X.-N. Wang, J.-C. Zhou, H.-X. Lou, J. Asian Nat. Prod. Res. 2013, 15, 473–481;
- 1eH. Shi, S. Yu, D. Liu, L. van Ofwegen, P. Proksch, W. Lin, Mar. Drugs 2012, 10, 1331–1344.
- 2For a review, see:
- 2aS. P. Roche, D. J. Aitken, Eur. J. Org. Chem. 2010, 5339–5358; For selected examples after 2010, see:
- 2bG. Singh, A. Meyer, J. Aubé, J. Org. Chem. 2014, 79, 452–458;
- 2cA. Jose, K. C. S. Lakshmi, E. Suresh, V. Nair, Org. Lett. 2013, 15, 1858–1861;
- 2dM. Ahmar, S. Thomé, B. Cazes, Eur. J. Org. Chem. 2012, 7093–7105;
- 2eB. Darses, I. N. Michaelides, F. Sladojevich, J. W. Ward, P. R. Rzepa, D. J. Dixon, Org. Lett. 2012, 14, 1684–1687;
- 2fM. Brasholz, B. Dugovič, H.-U. Reissig, Synthesis 2010, 3855–3864.
- 3
- 3aD. Fisher, L. I. Palmer, J. E. Cook, J. E. Davis, J. Read deAlaniz, Tetrahedron 2014, 70, 4105–4110;
- 3bC. Piutti, F. Quartieri, Molecules 2013, 18, 12290–12312;
- 3cJ. P. Henschke, Y. Liu, X. Huang, Y. Chen, D. Meng, L. Xia, X. Wei, A. Xie, D. Li, Q. Huang, T. Sun, J. Wang, X. Gu, X. Huang, L. Wang, J. Xiao, S. Qiu, Org. Process Res. Dev. 2012, 16, 1905–1916;
- 3dK. Ulbrich, P. Kreitmeier, O. Reiser, Synlett 2010, 2037–2040;
- 3eO. Nieto Faza, C. S. López, R. Álvarez, Á. R. de Lera, Chem. Eur. J. 2004, 10, 4324–4333;
- 3fG. Piancatelli, Heterocycles 1982, 19, 1735–1744;
- 3gG. Piancatelli, A. Scettri, S. Barbadoro, Tetrahedron Lett. 1976, 17, 3555–3558.
10.1016/S0040-4039(00)71357-8 Google Scholar
- 4
- 4aJ. Mulzer, G. Giester, M. Gilbert, Helv. Chim. Acta 2005, 88, 1560–1579;
- 4bE. Pinot, A. Guy, A.-L. Guyon, J.-C. Rossi, T. Durand, Tetrahedron: Asymmetry 2005, 16, 1893–1895;
- 4cS. Caddick, S. Khan, L. M. Frost, N. J. Smith, S. Cheung, G. Pairaudeau, Tetrahedron 2000, 56, 8953–8958;
- 4dG. Adembri, G. Giorgi, R. L. Lampariello, M. L. Paoli, A. Sega, J. Chem. Soc. Perkin Trans. 1 2000, 2649–2656;
- 4eS. Al-Busafi, M. G. B. Drew, T. Sanders, R. C. Whitehead, Tetrahedron Lett. 1998, 39, 1647–1650;
- 4fG. Piancatelli, M. D’Auria, F. D’Onofrio, Synthesis 1994, 867–889;
- 4gT. Shono, Y. Matsumura, H. Hamaguchi, K. Nakamura, Chem. Lett. 1976, 1249–1252.
- 5For furan photooxidations see:
- 5aC. S. Foote, M. T. Wuesthoff, S. Wexler, I. G. Burstain, R. Denny, G. O. Schenck, K. H. Schulte-Elte, Tetrahedron 1967, 23, 2583–2599;
- 5bK. Gollnick, A. Griesbeck, Angew. Chem. Int. Ed. Engl. 1983, 22, 726–727; Angew. Chem. 1983, 95, 751;
- 5cK. Gollnick, A. Griesbeck, Tetrahedron 1985, 41, 2057–2068;
- 5dB. L. Feringa, Recl. Trav. Chim. Pays-Bas 1987, 106, 469–488.
- 6aE. J. Corey, M. M. Mehrotra, J. Am. Chem. Soc. 1984, 106, 3384;
- 6bB. B. Snider, Z. Shi, J. Am. Chem. Soc. 1992, 114, 1790–1800;
- 6cB. B. Snider, Z. Shi, S. V. O’Neil, K. D. Kreutter, T. L. Arakaki, J. Org. Chem. 1994, 59, 1726–1729;
- 6dT.-C. Kao, G. J. Chuang, C.-C. Liao, Angew. Chem. Int. Ed. 2008, 47, 7325–7327; Angew. Chem. 2008, 120, 7435–7437.
- 7G. Vassilikogiannakis, M. Stratakis, Angew. Chem. Int. Ed. 2003, 42, 5465–5468; Angew. Chem. 2003, 115, 5623–5626.
- 8
- 8aT. Montagnon, D. Noutsias, I. Alexopoulou, M. Tofi, G. Vassilikogiannakis, Org. Biomol. Chem. 2011, 9, 2031–2039;
- 8bT. Montagnon, M. Tofi, G. Vassilikogiannakis, Acc. Chem. Res. 2008, 41, 1001–1011;
- 8cD. Noutsias, I. Alexopoulou, T. Montagnon, G. Vassilikogiannakis, Green Chem. 2012, 14, 601–604.
- 9For rearrangement of 2→3, see:
- 9aA. Scettri, G. Piancatelli, M. D’Auria, G. David, Tetrahedron 1979, 35, 135–138;
- 9bM. D’Auria, Heterocycles 2000, 52, 185–194, and references therein;
- 9cG. Stork, C. Kowalski, G. Garcia, J. Am. Chem. Soc. 1975, 97, 3258–3260.
- 10
- 10aS. Das, S. Chandrasekhar, J. S. Yadav, R. Grée, Chem. Rev. 2007, 107, 3286–3337;
- 10bP. W. Collins, S. W. Djuric, Chem. Rev. 1993, 93, 1533–1564;
- 10cK. C. Nicolaou, E. J. Sorensen, Classics in Total Synthesis, Wiley-VCH, Weinheim, 1996.
- 11L. Novák, C. Szántay, T. Meisel, J. Aszódi, É. Szabó, J. Fekete, Tetrahedron 1985, 41, 435–450.
- 12For recent selected examples, see:
- 12aJ.-Q. Liu, Y.-F. Yang, X.-Y. Li, E.-Q. Liu, Z.-R. Li, L. Zhou, Y. Li, M.-H. Qiu, Phytochemistry 2013, 96, 265–272;
- 12bR. Tohme, L. Al Aaraj, T. Ghaddar, H. Gali-Muhtasib, N. A. Saliba, N. Darwiche, Molecules 2013, 18, 8275–8288;
- 12cY. Li, M.-C. Zhu, M.-L. Zhang, Y.-F. Wang, M. Dong, Q.-W. Shi, C.-H. Huo, F. Sauriol, H. Kiyota, Y.-C. Gu, B. Cong, Tetrahedron Lett. 2012, 53, 2601–2603;
- 12dY. Deng, Y.-W. Chin, H.-B. Chai, E. C. de Blanco, L. B. S. Kardono, S. Riswan, D. D. Soejarto, N. R. Farnsworth, A. D. Kinghorn, Phytochem. Lett. 2011, 4, 213–217.
- 13For the isolation of Untenone A, see:
- 13aM. Ishibashi, S. Takeuchi, J. Kobayashi, Tetrahedron Lett. 1993, 34, 3749–3750; For a synthesis of Untenone A starting from furans, see:
- 13bS. Al-Busafi, R. C. Whitehead, Tetrahedron Lett. 2000, 41, 3467–3470.
- 14
- 14aM. S. Schechter, N. Green, F. B. Laforge, J. Am. Chem. Soc. 1949, 71, 3165–3173;
- 14bL. Crombie, M. Elliott, Fortschr. Chem. Org. Naturst. 1961, 19, 120–164;
- 14cG. Piancatelli, M. D’Auria, A. Scettri, U.S Pat. 4413145, 1981 [Chem. Abstr. 1982, 96, 217332b].
- 15
- 15aA. Kobayashi, K. Yamashita, K. Ohshima, I. Yamamoto, Agric. Biol. Chem. 1971, 35, 1961–1965;
- 15bP. R. Chadwich, Mosquito News 1970, 30, 162–170;
- 15cM. Matsui, H. Meguro, Agric. Biol. Chem. 1964, 28, 27–31.
- 16For selected examples, see:
- 16aR. J. Capon, S. Singh, S. Ali, S. Sotheeswaran, Aust. J. Chem. 2005, 58, 18–20;
- 16bR. S. Compagnone, I. C. Piña, H. R. Rangel, F. Dagger, A. I. Suárez, M. V. R. Reddy, D. J. Faulkner, Tetrahedron 1998, 54, 3057–3068.
- 17For the preparation of similar endoperoxy-mono-hemiketals, or endoperoxy-bis-ketals, via photooxidation of α,β-unsaturated ketones, or dienes, see; references [6b] and [6c].
- 18For more details see the Supporting Information.
- 19
- 19aS. Tsukamoto, S. Takeuchi, M. Ishibashi, J. Kobayashi, J. Org. Chem. 1992, 57, 5255–5260;
- 19bJ. Kobayashi, S. Tsukamoto, S. Takeuchi, M. Ishibashi, Tetrahedron 1993, 49, 5955–5960.
- 20S. Al-Busafi, J. R. Doncaster, M. G. B. Drew, A. C. Regan, R. C. Whitehead, J. Chem. Soc. Perkin Trans. 1 2002, 476–484.
- 21
- 21aT. Newhouse, P. S. Baran, R. W. Hoffmann, Chem. Soc. Rev. 2009, 38, 3010–3021;
- 21bT. Gaich, P. S. Baran, J. Org. Chem. 2010, 75, 4657–4673.