Enantio- and Diastereoselective Access to Distant Stereocenters Embedded within Tetrahydroxanthenes: Utilizing ortho-Quinone Methides as Reactive Intermediates in Asymmetric Brønsted Acid Catalysis†
M. Sc. Chien-Chi Hsiao
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen (Germany)
Search for more papers by this authorM. Sc. Hsuan-Hung Liao
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Magnus Rueping
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen (Germany)
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen (Germany)Search for more papers by this authorM. Sc. Chien-Chi Hsiao
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen (Germany)
Search for more papers by this authorM. Sc. Hsuan-Hung Liao
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Magnus Rueping
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen (Germany)
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen (Germany)Search for more papers by this authorH.-H.L. and C.-C.H. were supported by DAAD fellowships. We gratefully acknowledge financial support from the DFG.
Graphical Abstract
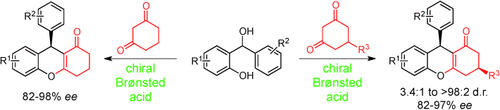
Procedures for the Brønsted acid catalyzed asymmetric synthesis of 9-substituted tetrahydroxanthenones and 3,9-disubstituted tetrahydroxanthenone derivatives have been developed. The procedures are based on the in situ generation of ortho-quinone methides and their subsequent reaction with 1,3-dicarbonyl compounds. The reaction provides products with a high level of asymmetric induction.
Abstract
A protocol for the highly enantioselective synthesis of 9-substituted tetrahydroxanthenones by means of asymmetric Brønsted acid catalysis has been developed. A chiral binol-based N-triflyphosphoramide was found to promote the in situ generation of ortho-quinone methides and their subsequent reaction with 1,3-cyclohexanedione to provide the desired products with excellent enantioselectivities. In addition, a highly enantio- and diastereoselective Brønsted acid catalyzed desymmetrization of 5-monosubstituted 1,3-dicarbonyl substrates with ortho-quinone methides gives rise to valuable tetrahydroxanthenes containing two distant stereocenters.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201406587_sm_miscellaneous_information.pdf2.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:
- 1aD. Morton, S. Leach, C. Cordier, S. Warriner, A. Nelson, Angew. Chem. Int. Ed. 2009, 48, 104–109; Angew. Chem. 2009, 121, 110–115;
- 1bF. E. Koehn, G. T. Carter, Nat. Rev. Drug Discovery 2005, 4, 206–220;
- 1cN. Harada, K. Nakanishi, Acc. Chem. Res. 1972, 5, 257–263.
- 2For reviews of chemistry and biology of chromenes, see:
- 2aM. Curini, G. Cravotto, F. Epifano, G. Giannone, Curr. Med. Chem. 2006, 13, 199–222;
- 2bG. P. Ellis, I. M. Lockhart, in The Chemistry of Heterocyclic Compounds, Chromenes, Chromanones, and Chromones, Vol. 31 (Ed.: ), Wiley-VCH, Weinheim, 2007, pp. 1–1196;
- 2c Chromenes, Chromanones, and Chromones. The Chemistry of Heterocyclic Compounds, Vol. 31 (Ed.: ), Wiley-Interscience, New York, 1977;
- 2dG. R. Green, J. M. Evans, A. K. Vong in Comprehensive Heterocyclic Chemistry II, Vol. 5 (Eds.: ), Pergamon, Oxford, 1995, pp. 469–473;
- 2eK. C. Nicolaou, J. A. Pfefferkorn, A. J. Roecker, G.-Q. Cao, S. Barluenga, H. J. Mitchell, J. Am. Chem. Soc. 2000, 122, 9939–9953.
- 3
- 3aT. Nishikata, Y. Yamamoto, N. Miyaura, Adv. Synth. Catal. 2007, 349, 1759–1764;
- 3bJ.-W. Xie, X. Huang, L.-P. Fan, D.-C. Xu, X.-X. Li, H. Su, Y.-H. Wang, Adv. Synth. Catal. 2009, 351, 3077–3082;
- 3cX. Zhang, S. Zjang, W. Wang, Angew. Chem. Int. Ed. 2010, 49, 1481–1484; Angew. Chem. 2010, 122, 1523–1526;
- 3dM. Terada, T. Yamanaka, Y. Toda, Chem. Eur. J. 2013, 19, 13658–13662;
- 3eC.-C. Hsiao, H.-H. Liao, E. Sugiono, I. Atodiresei, M. Rueping, Chem. Eur. J. 2013, 19, 9775–9779.
- 4K. Fries, Justus Liebigs Ann. Chem. 1907, 339, 350–356.
- 5For reviews of o-QMs, see:
- 5aN. J. Willis, C. D. Bray, Chem. Eur. J. 2012, 18, 9160–9173;
- 5b Quinone Methides (Ed.: ), Wiley, New York, 2009, p. 412;
- 5cR. W. Van De Water, T. R. R. Pettus, Tetrahedron 2002, 58, 5367–5405;
- 5dT. P. Pathak, M. S. Sigman, J. Org. Chem. 2011, 76, 9210–9215;
- 5eC. M. Beaudry, J. P. Malerich, D. Trauner, Chem. Rev. 2005, 105, 4757–4778.
- 6Recent examples for o-QMs in total synthesis:
- 6aT. H. Jepsen, S. B. Thomas, Y. Lin, C. I. Sathais, I. de Miguel, S. A. Snyder, Angew. Chem. Int. Ed. 2014, 53, 6747–6751; Angew. Chem. 2014, 126, 6865–6869;
- 6bD. H. Liao, H. H. Li, X. G. Lei, Org. Lett. 2012, 14, 18–21;
- 6cJ. C. Green, E. R. Brown, T. R. R. Pettus, Org. Lett. 2012, 14, 2929–2931;
- 6dJ. C. Green, S. Jiminez-Alonso, E. R. Brown, T. R. R. Pettus, Org. Lett. 2011, 13, 5500–5503;
- 6eA. L. Lawrence, R. M. Adlington, J. E. Baldwin, V. Lee, J. A. Kershaw, A. T. Thompson, Org. Lett. 2010, 12, 1676–1679;
- 6fD. R. Smith, S. B. Herzon, J. Am. Chem. Soc. 2010, 132, 2540–2541;
- 6gC. F. Bender, F. K. Yoshimoto, C. L. Paradise, J. K. De Brabander, J. Am. Chem. Soc. 2009, 131, 11350–11352.
- 7For selected examples for o-QMs generation by oxidation:
- 7aL. M. Bishop, M. Winkler, K. N. Houk, R. G. Bergman, D. Trauner, Chem. Eur. J. 2008, 14, 5405–5408; thermal:
- 7bH. Sugimoto, S. Nakamura, T. Ohwada, J. Org. Chem. 2007, 72, 10088–10095; photo:
- 7cY. Chen, M. G. Steinmetz, J. Org. Chem. 2006, 71, 6053–6060; base:
- 7dA. E. Mattson, K. A. Scheidt, J. Am. Chem. Soc. 2007, 129, 4508–4509; acid:
- 7eP. Batsomboon, W. Phakhodee, S. Ruchirawat, P. Ploypradith, J. Org. Chem. 2009, 74, 4009–4012.
- 8
- 8aR. Jana, T. P. Pathak, K. H. Jensen, M. S. Sigman, Org. Lett. 2012, 14, 4074–4077;
- 8bT. P. Pathak, M. S. Sigman, Org. Lett. 2011, 13, 2774–2777;
- 8cT. P. Pathak, K. M. Gligorich, B. E. Welm, M. S. Sigman, J. Am. Chem. Soc. 2010, 132, 7870–7871;
- 8dK. H. Jensen, J. D. Webb, M. S. Sigman, J. Am. Chem. Soc. 2010, 132, 17471–17482;
- 8eK. H. Jensen, T. P. Pathak, Y. Zhang, M. S. Sigman, J. Am. Chem. Soc. 2009, 131, 17074–17075;
- 8fY. Zhang, M. S. Sigman, J. Am. Chem. Soc. 2007, 129, 3076–3077.
- 9S. B. Ferreira, F. C. da Silva, A. C. Pinto, D. T. G. Gonzaga, V. F. Ferreira, J. Heterocycl. Chem. 2009, 46, 1080–1097.
- 10E. Alden-Danforth, M. T. Scerba, T. Lectka, Org. Lett. 2008, 10, 4951–4953.
- 11Y. Luan, S. E. Schaus, J. Am. Chem. Soc. 2012, 134, 19965–19968.
- 12
- 12aH. Lv, W. Q. Jia, L. H. Sun, S. Ye, Angew. Chem. Int. Ed. 2013, 52, 8607–8610; Angew. Chem. 2013, 125, 8769–8772;
- 12bJ. Izquierdo, A. Orue, K. A. Scheidt, J. Am. Chem. Soc. 2013, 135, 10634–10637.
- 13For reviews of Brønsted acid catalysis, see:
- 13aM. Mahlau, B. List, Angew. Chem. Int. Ed. 2013, 52, 518–533; Angew. Chem. 2013, 125, 540–556;
- 13bR. J. Phipps, G. L. Hamilton, F. D. Toste, Nat. Chem. 2012, 4, 603–614;
- 13cM. Rueping, A. Kuenkel, I. Atodiresei, Chem. Soc. Rev. 2011, 40, 4539–4549;
- 13dS. Schenker, A. Zamfir, M. Freund, S. B. Tsogoeva, Eur. J. Org. Chem. 2011, 2209–2222;
- 13eM. Terada, Curr. Org. Chem. 2011, 15, 2227–2256;
- 13fD. Kampen, C. M. Reisinger, B. List, Top. Curr. Chem. 2010, 291, 395–456;
- 13gM. Terada, Synthesis 2010, 1929–1982;
- 13hA. Zamfir, S. Schenker, M. Freund, S. B. Tsogoeva, Org. Biomol. Chem. 2010, 8, 5262–5276;
- 13iT. Akiyama, Chem. Rev. 2007, 107, 5744–5758;
- 13jD. Parmar, E. Sugiono, S. Raja, M. Rueping, Chem. Rev. 2014, DOI: .
- 14For pioneering work in the field of chiral binol-based N-triflylphosphoramides, see:
- 14aS. A. Yamamoto, D. Nakashima, H. Yamamoto, J. Am. Chem. Soc. 2006, 128, 9626–9627;
- 14bP. Jiao, D. Nakashima, H. Yamamoto, Angew. Chem. Int. Ed. 2008, 47, 2411–2413; Angew. Chem. 2008, 120, 2445–2447; for an overview, see:
- 14cM. Rueping, B. J. Nachtsheim, W. Ieawsuwan, I. Atodiresei, Angew. Chem. Int. Ed. 2011, 50, 6706–6720; Angew. Chem. 2011, 123, 6838–6853;
- 14dM. Fleischmann, D. Drettwann, E. Sugiono, M. Rueping, R. M. Gschwind, Angew. Chem. Int. Ed. 2011, 50, 6364–6369; Angew. Chem. 2011, 123, 6488–6493;
- 14eK. Kaupmees, N. Tolstoluzhsky, S. Raja, M. Rueping, I. Leito, Angew. Chem. Int. Ed. 2013, 52, 11569–11572; Angew. Chem. 2013, 125, 11783–11786; selected examples from our group, see:
- 14fM. Rueping, W. Ieawsuwan, A. P. Antonchick, B. J. Nachtsheim, Angew. Chem. Int. Ed. 2007, 46, 2097–2100; Angew. Chem. 2007, 119, 2143–2146;
- 14gM. Rueping, B. J. Nachtsheim, S. A. Moreth, M. Bolte, Angew. Chem. Int. Ed. 2008, 47, 593–596; Angew. Chem. 2008, 120, 603–606;
- 14hM. Rueping, T. Theissmann, A. Kuenkel, R. M. Koenigs, Angew. Chem. Int. Ed. 2008, 47, 6798–6801; Angew. Chem. 2008, 120, 6903–6906;
- 14iM. Rueping, W. Ieawsuwan, Chem. Commun. 2011, 47, 11450–11452;
- 14jM. Rueping, B. J. Nachtsheim, R. M. Koenigs, W. Ieawsuwan, Chem. Eur. J. 2010, 16, 13116–13126;
- 14kM. Rueping, U. Uria, M.-Y. Lin, I. Atodiresei, J. Am. Chem. Soc. 2011, 133, 3732–3735;
- 14lM. Rueping, M. S. Maji, H. B. Küçük, I. Atodiresei, Angew. Chem. Int. Ed. 2012, 51, 12864–12868; Angew. Chem. 2012, 124, 13036–13040;
- 14mS. Raja, W. Ieawsuwan, V. Korotkov, M. Rueping, Chem. Asian J. 2012, 7, 2361–2366.
- 15
- 15aM. Freccero, C. Di Valentin, M. Sarzi-Amade, J. Am. Chem. Soc. 2003, 125, 3544–3553;
- 15bC. Di Valentin, M. Freccero, R. Zanaletti, M. Sarzi-Amade, J. Am. Chem. Soc. 2001, 123, 8366–8377.
- 16For selected examples of desymmetrization by chiral phosphoric acids, see:
- 16aZ. Wang, Z. Chen, J. Sun, Angew. Chem. Int. Ed. 2013, 52, 6685–6688; Angew. Chem. 2013, 125, 6817–6820;
- 16bK. Mori, Y. Ichikawa, M. Kobayashi, Y. Shibata, M. Yamanaka, T. Akiyama, J. Am. Chem. Soc. 2013, 135, 3964–3970;
- 16cG. Qabaja, J. E. Wilent, A. R. Benavides, G. E. Bullard, K. S. Petersen, Org. Lett. 2013, 15, 1266–1269;
- 16dD. M. Rubush, M. A. Morges, B. J. Rose, D. H. Thamm, T. Rovis, J. Am. Chem. Soc. 2012, 134, 13554–13557;
- 16eS. Müller, M. J. Webber, B. List, J. Am. Chem. Soc. 2011, 133, 18534–18537;
- 16fV. N. Wakchaure, B. List, Angew. Chem. Int. Ed. 2010, 49, 4136–4139; Angew. Chem. 2010, 122, 4230–4233;
- 16gQ. Gu, Z.-Q. Rong, C. Zheng, S.-L. You, J. Am. Chem. Soc. 2010, 132, 4056–4057;
- 16hE. B. Rowland, G. B. Rowland, E. Rivera-Otero, J. C. Antilla, J. Am. Chem. Soc. 2007, 129, 12084–12085.
- 17For reviews, see:
- 17aJ. Clayden, Chem. Soc. Rev. 2009, 38, 817–829;
- 17bK. Mikami, M. Shimizu, H.-C. Zhang, B. E. Maryanoff, Tetrahedron 2001, 57, 2917–2951;
- 17cH. Sailes, A. Whiting, J. Chem. Soc. Perkin Trans. 1 2000, 1785–1805;
- 17dA. H. Hoveyda, D. A. Evans, G. C. Fu, Chem. Rev. 1993, 93, 1307–1370.
- 18For selected examples, see:
- 18aY. Hayashi, H. Yamaguchi, M. Toyoshima, K. Okado, T. Toyo, M. Shoji, Chem. Eur. J. 2010, 16, 10150–10159;
- 18bR. Hayashi, M. C. Walton, R. P. Hsung, J. H. Schwab, X. L. Yu, Org. Lett. 2010, 12, 5768–5771;
- 18cY. Liang, L. Wang, R. Zhu, L. Deng, Y. Yang, J. Quan, J. Chen, Z. Yang, Adv. Synth. Catal. 2010, 352, 2387–2393;
- 18dY. Nishigaichi, T. Fujimoto, A. Takuwa, H. Iwamoto, Tetrahedron Lett. 2010, 51, 6298–6300;
- 18eJ. Clayden, L. Lemigre, G. A. Morris, M. Pickworth, T. J. Snape, L. H. Jones, J. Am. Chem. Soc. 2008, 130, 15193–15202;
- 18fJ. Clayden, A. Lund, L. Vallverdú, M. Helliwell, Nature 2004, 431, 966–971;
- 18gS. Shirokawa, M. Kamiyama, T. Nakamura, M. Okada, A. Nakazaki, S. Hosokawa, S. Kobayashi, J. Am. Chem. Soc. 2004, 126, 13604–13605.
- 19See the Supporting Information for details.
- 20A. Kumar, S. Sharma, R. A. Maurya, J. Sarkar, J. Comb. Chem. 2010, 12, 20–24.
- 21During the preparation of this manuscript, a similar protocol appeared: O. El-Sepelgy, S. Haseloff, S. K. Alamsetti, C. Schneider, Angew. Chem. Int. Ed. 2014, 53, 7923–7927; Angew. Chem. 2014, 126, 8057–8061.
- 22Recent reviews for the application of organocatalysis in the synthesis of bioactive molecules and natural products:
- 22aR. Marcia de Figueiredo, M. Christmann, Eur. J. Org. Chem. 2007, 2575–2600;
- 22bE. Marqués-López, R. P. Herrera, M. Christmann, Nat. Prod. Rep. 2010, 27, 1138–1167.