Furan-Based o-Quinodimethanes by Gold-Catalyzed Dehydrogenative Heterocyclization of 2-(1-Alkynyl)-2-alken-1-ones: A Modular Entry to 2,3-Furan-Fused Carbocycles†
Liejin Zhou
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, Department of Chemistry, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200062 (P.R. China) http://faculty.ecnu.edu.cn/s/1811/t/20975/main.jspy
Search for more papers by this authorMingrui Zhang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, Department of Chemistry, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200062 (P.R. China) http://faculty.ecnu.edu.cn/s/1811/t/20975/main.jspy
Search for more papers by this authorDr. Wenbo Li
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, Department of Chemistry, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200062 (P.R. China) http://faculty.ecnu.edu.cn/s/1811/t/20975/main.jspy
Search for more papers by this authorCorresponding Author
Prof. Dr. Junliang Zhang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, Department of Chemistry, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200062 (P.R. China) http://faculty.ecnu.edu.cn/s/1811/t/20975/main.jspy
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, Department of Chemistry, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200062 (P.R. China) http://faculty.ecnu.edu.cn/s/1811/t/20975/main.jspySearch for more papers by this authorLiejin Zhou
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, Department of Chemistry, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200062 (P.R. China) http://faculty.ecnu.edu.cn/s/1811/t/20975/main.jspy
Search for more papers by this authorMingrui Zhang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, Department of Chemistry, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200062 (P.R. China) http://faculty.ecnu.edu.cn/s/1811/t/20975/main.jspy
Search for more papers by this authorDr. Wenbo Li
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, Department of Chemistry, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200062 (P.R. China) http://faculty.ecnu.edu.cn/s/1811/t/20975/main.jspy
Search for more papers by this authorCorresponding Author
Prof. Dr. Junliang Zhang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, Department of Chemistry, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200062 (P.R. China) http://faculty.ecnu.edu.cn/s/1811/t/20975/main.jspy
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, Department of Chemistry, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200062 (P.R. China) http://faculty.ecnu.edu.cn/s/1811/t/20975/main.jspySearch for more papers by this authorFinancial support from the National Natural Science Foundation of China (21372084), 973 Programs (2011CB808600), and the Changjiang Scholars and Innovative Research Team at the University (PCSIRT) is greatly appreciated. We thank Prof. Shengming Ma for helpful discussions.
Graphical Abstract
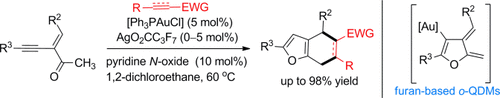
Caught in a trap: A novel strategy for in situ generation of furan-based ortho-quinodimethanes (o-QDMs) using the title reaction was developed. These furan-based o-QDMs were trapped by electron-deficient olefins and alkynes, thus leading to various 2,3-furan-fused carbocycles in good yields with high diastereo- and regioselectivities. EWG=electron-withdrawing group.
Abstract
A strategy for in situ generation of furan-based ortho-quinodimethanes (o-QDMs) by the gold(I)-mediated dehydrogenative heterocyclization of 2-(1-alkynyl)-2-alken-1-ones in the presence of pyridine N-oxide under mild reaction conditions was developed. These in situ furan-based o-QDMs were trapped by electron-deficient olefins and alkynes, thus furnishing various 2,3-furan-fused carbocycles in good yields with high diastereo- and regioselectivities.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201403709_sm_miscellaneous_information.pdf4.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1B. A. Keay, J. M. Hopkins, P. W. Dibble in Comprehensive Heterocyclic Chemistry III (Eds.: ), Elsevier, Oxoford, 2008, chap. 3.08.
- 2
- 2aD. L. Wright, Prog. Heterocycl. Chem. 2005, 17, 1–32;
- 2bH. N. C. Wong, K.-S. Yeung, Z. Yang in Comprehensive Heterocyclic Chemistry III (Eds.: ), Elsevier, Oxford, 2008, chap. 3.06.
- 3For recent reviews on furan synthesis, see:
- 3aA. S. K. Hashmi, Chem. Rev. 2007, 107, 3180–3211;
- 3bN. T. Patil, Y. Yamamoto, ARKIVOC 2007, 121–141;
- 3cA. Corma, A. Leyva-Pérez, M. J. Sabater, Chem. Rev. 2011, 111, 1657–1712;
- 3dC. Winter, N. Krause, Chem. Rev. 2011, 111, 1994–2009;
- 3eW. J. Moran, A. Rodríguez, Org. Prep. Proced. Int. 2012, 44, 103–130;
- 3fA. V. Gulevich, A. S. Dudnik, N. Chernyak, V. Gevorgyan, Chem. Rev. 2013, 113, 3084–3213.
- 4For selected recent examples of gold-catalyzed synthesis of furans:
- 4aG. Zhang, X. Huang, G. Li, L. Zhang, J. Am. Chem. Soc. 2008, 130, 1814–1815;
- 4bA. Aponick, C.-Y. Li, J. Malinge, E. F. Marques, Org. Lett. 2009, 11, 4624–4627;
- 4cM. Egi, K. Azechi, S. Akai, Org. Lett. 2009, 11, 5002–5005;
- 4dS. Kramer, J. L. H. Madsen, M. Rottländer, T. Skrydstrup, Org. Lett. 2010, 12, 2758–2761;
- 4eA. Dudnik, Y. Xia, Y. Li, V. Gevorgyan, J. Am. Chem. Soc. 2010, 132, 7645–7655;
- 4fT. Wang, S. Shi, M. M. Hansmann, E. Rettenmeier, M. Rudolph, A. S. K. Hashmi, Angew. Chem. 2014, 126, 3789–3793; Angew. Chem. Int. Ed. 2014, 53, 3715–3719; for synthesis of 2,3-furan-fused carbocyles:
- 4gA. S. K. Hashmi, P. Haufe, C. Schmid, A. Rivas Nass, W. Frey, Chem. Eur. J. 2006, 12, 5376–5382;
- 4hA. S. K. Hashmi, M. C. Blanco, E. Kurpejović, W. Frey, J. W. Bats, Adv. Synth. Catal. 2006, 348, 709–713;
- 4iZ. Dong, C.-H. Liu, Y. Wang, M. Lin, Z.-X. Yu, Angew. Chem. 2013, 125, 14407–14411; Angew. Chem. Int. Ed. 2013, 52, 14157–14161, and references therein.
- 5
- 5aV. Benešová, Z. Samek, V. Herout, F. Šorm, Collect. Czech. Chem. Commun. 1969, 34, 582–592;
- 5bH. Tazaki, H. Soutome, K. Nabeta, H. Okuyama, H. Becker, Phytochemistry 1996, 42, 465–468;
- 5cO. Richou, V. Vaillancourt, D. J. Faulkner, K. F. Albizati, J. Org. Chem. 1989, 54, 4729–4730;
- 5dP. Torres, J. Ayala, C. Grande, J. Anaya, M. Grande, Phytochemistry 1999, 52, 1507–1513;
- 5eE. P. Kündig, R. Cannas, M. Laxmisha, L. Ronggang, S. Tchertchian, J. Am. Chem. Soc. 2003, 125, 5642–5643.
- 6For reviews on o-QDMs:
- 6aN. Martín, C. Seoane, M. Hanack, Org. Prep. Proc. Int. 1991, 23, 237–272;
- 6bH. Nemoto, K. Fukumoto, Tetrahedron 1998, 54, 5425–5464;
- 6cJ. L. Segura, N. Martín, Chem. Rev. 1999, 99, 3199–3246; for the first example of o-QDM, see:
- 6dM. P. Cava, D. R. Napier, J. Am. Chem. Soc. 1957, 79, 1701–1705; for recent examples, see:
- 6eB. Grosch, C. N. Orlebar, E. Herdtweck, W. Massa, T. Bach, Angew. Chem. 2003, 115, 3822–3824;
10.1002/ange.200351567 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 3693–3696;
- 6fA. Ouchi, Z. Li, M. Sakuragi, T. Majima, J. Am. Chem. Soc. 2003, 125, 1104–1108;
- 6gY. Matsuya, N. Ohsawa, H. Nemoto, J. Am. Chem. Soc. 2006, 128, 412–413;
- 6hR. Kuwano, T. Shige, J. Am. Chem. Soc. 2007, 129, 3802–3803;
- 6iH. Yoshida, S. Nakano, M. Mukae, J. Ohshita, Org. Lett. 2008, 10, 4319–4322;
- 6jS. Ueno, M. Ohtsubo, R. Kuwano, J. Am. Chem. Soc. 2009, 131, 12904–12905;
- 6kH. Yoshida, M. Mukae, J. Ohshita, Chem. Commun. 2010, 46, 5253–5255;
- 6lS. Ueno, M. Ohtsubo, R. Kuwano, Org. Lett. 2010, 12, 4332–4334;
- 6mS. F. Zhu, R. X. Liang, H. F. Jiang, W. Q. Wu, Angew. Chem. 2012, 124, 11019–11023; Angew. Chem. Int. Ed. 2012, 51, 10861–10865.
- 7For general reviews on heteroaromatic oQDMs, see:
- 7aT. S. Chou, Rev. Heteroat. Chem. 1993, 8, 65–104;
- 7bS. J. Collier, R. C. Storr in Progress in Heterocyclic Chemistry, Vol. 10 (Eds.: ), Pergamon, New York, 1998, pp. 25–48; Magnus and co-workers have done seminal work in developing an indole-2,3-quinodimethane strategy in total synthesis. See:
- 7cP. Magnus, T. Gallagher, P. Brown, P. Pappalardo, Acc. Chem. Res. 1984, 17, 35–41; for a review of indole-2,3-quinodimethanes, see:
- 7dU. Pindur, H. Erfanian-Abdoust, Chem. Rev. 1989, 89, 1681–1689; for recent examples, see:
- 7eN. Kuroda, Y. Takahashi, K. Yoshinaga, C. Mukai, Org. Lett. 2006, 8, 1843–1845;
- 7fY. Liu, M. Nappi, E. Arceo, S. Vera, P. Melchiorre, J. Am. Chem. Soc. 2011, 133, 15212–15218;
- 7gY.-C. Xiao, Q.-Q. Zhou, L. Dong, T.-Y. Liu, Y.-C. Chen, Org. Lett. 2012, 14, 5940–5943;
- 7hS. Zhao, R. B. Andrade, J. Am. Chem. Soc. 2013, 135, 13334–13337.
- 8G.-B. Liu, H. Mori, S. Katsumura, Chem. Commun. 1996, 2251–2252.
- 9Trahanovsky and co-workers have done seminal work on the generation and dimerization of furan-based oQDMs:
- 9aW. S. Trahanovsky, T. J. Cassady, T. L. Woods, J. Am. Chem. Soc. 1981, 103, 6691–6695;
- 9bC.-H. Chou, W. S. Trahanovsky, J. Am. Chem. Soc. 1986, 108, 4138–4144;
- 9cM.-K. Leung, W. S. Trahanovsky, J. Am. Chem. Soc. 1995, 117, 841–850.
- 10For metal-catalyzed reactions, see:
- 10aT. Yao, X. Zhang, R. C. Larock, J. Am. Chem. Soc. 2004, 126, 11164–11165;
- 10bN. T. Patil, H. Wu, Y. Yamamoto, J. Org. Chem. 2005, 70, 4531–4534; for selected work from our group, see:
- 10cY. Xiao, J. Zhang, Angew. Chem. 2008, 120, 1929–1932; Angew. Chem. Int. Ed. 2008, 47, 1903–1906;
- 10dF. Liu, D. Qian, L. Li, X. Zhao, J. Zhang, Angew. Chem. 2010, 122, 6819–6822; Angew. Chem. Int. Ed. 2010, 49, 6669–6672;
- 10eZ.-M. Zhang, P. Chen, W. Li, Y. Niu, X. L. Zhao, J. Zhang, Angew. Chem. 2014, 126, 4439–4443; Angew. Chem. Int. Ed. 2014, 53, 4350–4354;
- 10fG. Zhou, F. Liu, J. Zhang, Chem. Eur. J. 2011, 17, 3101–3104.
- 11Selected transformations without a metal catalyst, see:
- 11aY. H. Liu, S. Zhou, Org. Lett. 2005, 7, 4609–4611;
- 11bT. Yao, X. Zhang, R. C. Larock, J. Org. Chem. 2005, 70, 7679–7686;
- 11cL. Zhao, F. Xie, G. Cheng, Y. Hu, Angew. Chem. 2009, 121, 6642–6645; Angew. Chem. Int. Ed. 2009, 48, 6520–6523;
- 11dW. Li, J. Zhang, Org. Lett. 2014, 16, 162–165, and references therein.
- 12
- 12aY. Wang, A. Yepremyan, S. Ghorai, R. Todd, D. H. Aue, L. Zhang, Angew. Chem. 2013, 125, 7949–7953; Angew. Chem. Int. Ed. 2013, 52, 7795–7799;
- 12bL. Ye, Y. Wang, D. H. Aue, L. Zhang, J. Am. Chem. Soc. 2012, 134, 31–34.
- 13C.-M. Ting, Y.-L. Hsu, R.-S. Liu, Chem. Commun. 2012, 48, 6577–6579.
- 14CCDC 984637 (3 b), 984638 (7), 984633 (10 b), 984634 (12 a), and 984635 (13) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.