Heptagon-Embedded Pentacene: Synthesis, Structures, and Thin-Film Transistors of Dibenzo[d,d′]benzo[1,2-a:4,5-a′]dicycloheptenes†
Xuejin Yang
Department of Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong (China)
Search for more papers by this authorDanqing Liu
Department of Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong (China)
Search for more papers by this authorCorresponding Author
Prof. Qian Miao
Department of Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong (China)
Institute of Molecular Functional Materials (Areas of Excellence Scheme, University Grants Committee), Hong Kong (China)
Department of Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong (China)Search for more papers by this authorXuejin Yang
Department of Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong (China)
Search for more papers by this authorDanqing Liu
Department of Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong (China)
Search for more papers by this authorCorresponding Author
Prof. Qian Miao
Department of Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong (China)
Institute of Molecular Functional Materials (Areas of Excellence Scheme, University Grants Committee), Hong Kong (China)
Department of Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong (China)Search for more papers by this authorWe thank Hoi Shan Chan (the Chinese University of Hong Kong) for the single-crystal X-ray crystallography. This work was supported by the Research Grants Council of Hong Kong (project number: GRF402412) and the University Grants Committee of Hong Kong (project number: AoE/P-03/08).
Graphical Abstract
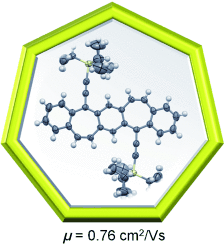
All sixes and sevens: A new class of conjugated polycyclic molecules have a nearly flat C6-C7-C6-C7-C6 polycyclic framework with a p-quinodimethane core. With a field-effect mobility of up to 0.76 cm2 V−1 s−1 as measured from solution-processed thin-film transistors, these molecules are alternatives to the pentacene analogues for application in organic electronic devices.
Abstract
This study presents a new class of conjugated polycyclic molecules that contain seven-membered rings, detailing their synthesis, crystal structures and semiconductor properties. These molecules have a nearly flat C6-C7-C6-C7-C6 polycyclic framework with a p-quinodimethane core. With field-effect mobilities of up to 0.76 cm2 V−1 s−1 as measured from solution-processed thin-film transistors, these molecules are alternatives to the well-studied pentacene analogues for applications in organic electronic devices.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201403509_sm_miscellaneous_information.pdf6.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent examples of conjugated polycyclic hydrocarbons containing four- and eight-membered rings, see:
- 1aBharat, R. Bhola, T. Bally, A. Valente, M. K. Cyrański, Ł. Dobrzycki, S. M. Spain, P. Rempała, M. R. Chin, B. T. King, Angew. Chem. 2010, 122, 409–412;
10.1002/ange.200905633 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 399–402;
- 1bC.-N. Feng, M.-Y. Kuo, Y.-T. Wu, Angew. Chem. 2013, 125, 7945–7948; Angew. Chem. Int. Ed. 2013, 52, 7791–7794.
- 2
- 2aY. T. Wu, J. S. Siegel, Chem. Rev. 2006, 106, 4843–4867;
- 2bL. T. Scott, Polycyclic Aromat. Compd. 2010, 30, 247–259.
- 3For recent examples, see:
- 3aB. D. Steinberg, E. A. Jackson, A. S. Filatov, A. Wakamiya, M. A. Petrukhina, L. T. Scott, J. Am. Chem. Soc. 2009, 131, 10537–10545;
- 3bT. Amaya, T. Nakata, T. Hirao, J. Am. Chem. Soc. 2009, 131, 10810–10811;
- 3cA. C. Whalley, K. N. Plunkett, A. A. Gorodetsky, C. L. Schenck, C.-Y. Chiu, M. L. Steigerwald, C. Nuckolls, Chem. Sci. 2011, 2, 132;
- 3dT. Amaya, T. Hirao, Chem. Commun. 2011, 47, 10524–10535;
- 3eT.-C. Wu, M.-K. Chen, Y.-W. Lee, M.-Yu. Kuo, Y.-T. Wu, Angew. Chem. 2013, 125, 1327–1331; Angew. Chem. Int. Ed. 2013, 52, 1289–1293.
- 4P. W. Rabideau, A. Sygula, Acc. Chem. Res. 1996, 29, 235–242.
- 5
- 5aF. G. Brunetti, X. Gong, M. Tong, A. J. Heeger, F. Wudl, Angew. Chem. 2010, 122, 542–546;
10.1002/ange.200905117 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 532–536;
- 5bD. T. Chase, A. G. Fix, S. J. Kang, B. D. Rose, C. D. Weber, Y. Zhong, L. N. Zakharov, M. C. Lonergan, C. Nuckolls, H. M. Haley, J. Am. Chem. Soc. 2012, 134, 10349–10352;
- 5cJ. Nishida, S. Tsukaguchi, Y. Yamashita, Chem. Eur. J. 2012, 18, 8964–8970;
- 5dJ. D. Wood, J. L. Jellison, A. D. Finke, L. Wang, K. N. Plunkett, J. Am. Chem. Soc. 2012, 134, 15783–15789;
- 5eJ. L. Jellison, C.-H. Lee, X. Zhu, J. D. Wood, K. N. Plunkett, Angew. Chem. 2012, 124, 12487–12490;
10.1002/ange.201206145 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 12321–12324;
- 5fH. Xia, D. Liu, X. Xu, Q. Miao, Chem. Commun. 2013, 49, 4301–4303.
- 6
- 6aK. Yamamoto, T. Harada, M. Nakazaki, J. Am. Chem. Soc. 1983, 105, 7171–7172;
- 6bK. Yamamoto, T. Harada, Y. Okamoto, H. Chikamatsu, M. Nakazaki, Y. Kai, T. Nakao, M. Tanaka, S. Harada, N. Kasai, J. Am. Chem. Soc. 1988, 110, 3578–3584;
- 6cK. Yamamoto, Y. Saitho, D. Iwaki, T. Ooka, Angew. Chem. 1991, 103, 1202–1203; Angew. Chem. Int. Ed. Engl. 1991, 30, 1173–1174;
- 6dJ. Luo, X. Xu, R. Mao, Q. Miao, J. Am. Chem. Soc. 2012, 134, 13796–13803;
- 6eK. Kawasumi, Q. Zhang, Y. Segawa, L. T. Scott, K. Itami, Nat. Chem. 2013, 5, 739–744.
- 7H. Xia, D. Liu, K. Song, Q. Miao, Chem. Sci. 2011, 2, 2402–2406.
- 8Dibenzo[d,d′]benzo[1,2-a:4,5-a′]dicycloheptene is the name recommended by the ACS. According to recent IUPAC nomenclature (1998), it should be named as dibenzo[d,d′]benzo[1,2-a:4,5-a′]di[7]annulene.
- 9A similar pentacyclic conjugated backbone of boron-substituted heteroarenes has two fewer π-electrons than molecules 1 a–c. See: A. J. Caruso, M. A. Siegler, J. D. Tovar, Angew. Chem. 2010, 122, 4309–4313; Angew. Chem. Int. Ed. 2010, 49, 4213–4217.
- 10
- 10aD. T. Chase, B. D. Rose, S. P. McClintock, L. N. Zakharov, M. M. Haley, Angew. Chem. 2011, 123, 1159–1162; Angew. Chem. Int. Ed. 2011, 50, 1127–1130;
- 10bD. T. Chase, A. G. Fix, B. D. Rose, C. D. Weber, S. Nobusue, C. E. Stockwell, L. N. Zakharov, M. C. Lonergan, M. M. Haley, Angew. Chem. 2011, 123, 11299–11302;
10.1002/ange.201104797 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 11103–11106;
- 10cA. Shimizu, Y. Tobe, Angew. Chem. 2011, 123, 7038–7042; Angew. Chem. Int. Ed. 2011, 50, 6906–6910.
- 11R. Umeda, S. Miyake, Y. Nishiyama, Chem. Lett. 2012, 41, 215–217.
- 12
- 12aM. C. Bonifacio, C. R. Robertson, J.-J. Jung, B. T. King, J. Org. Chem. 2005, 70, 8522–8526;
- 12bZ. Shi, X. Zhang, G. Yang, Z. Su, Z. Cui, Tetrahedron 2011, 67, 4110–4117.
- 13I. Agranat, D. Avnir, J. Chem. Soc. Perkin Trans. 1 1974, 1155–1161.
- 14CCDC 992241 (1 a) and 992242 (1 b) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15The typical bond length for a single bond between two sp2-hybridized carbon atoms is 1.45–1.46 Å. See: E. V. Anslyn, D. A. Dougherty, Modern Physical Organic Chemistry, University Science Books, Sausalito, 2004, chap. 1, p. 22.
- 16
- 16aJ. E. Anthony, J. S. Brooks, D. L. Eaton, S. R. Parkin, J. Am. Chem. Soc. 2001, 123, 9482–9483.
- 17The commonly used HOMO energy level of ferrocene is −4.80 eV, which is calculated on the basis of an approximation, neglecting solvent effects, using a work function of 4.6 eV for the standard hydrogen electrode (SHE) and an electrochemical potential of 0.2 V for the ferrocene/ferrocenium couple versus SHE. See:
- 17aJ. Pommerehne, H. Vestweber, W. Guss, R. F. Mahrt, H. Bässler, M. Porsch, J. Daub, Adv. Mater. 1995, 7, 551–554;
- 17bB. W. D’Andrade, S. Datta, S. R. Forrest, P. Djurovich, E. Polikarpov, M. E. Thompson, Org. Electron. 2005, 6, 11–20.
- 18Z. Liang, Q. Tang, J. Xu, Q. Miao, Adv. Mater. 2011, 23, 1535–1539.
- 19
- 19aM. M. Payne, S. R. Parkin, J. E. Anthony, C.-C. Kuo, T. N. J. Jackson, J. Am. Chem. Soc. 2005, 127, 4986–4987;
- 19bS. K. Park, T. N. Jackson, J. E. Anthony, D. A. Mourey, Appl. Phys. Lett. 2007, 91, 063514.
- 20G. Giri, E. Verploegen, S. C. B. Mannsfeld, S. Atahan-Evrenk, D. H. Kim, S. Y. Lee, H. A. Becerril, A. Aspuru-Guzik, M. F. Toney, Z. Bao, Nature 2011, 480, 504–508.
- 21S. Kobayashi, T. Nishikawa, T. Takenobu, S. Mori, T. Shimoda, T. Mitani, H. Shimotani, N. Yoshimoto, S. Ogawa, Y. Iwasa, Nat. Mater. 2004, 3, 317–322.