Enzyme-Catalyzed Oxidation of 5-Hydroxymethylfurfural to Furan-2,5-dicarboxylic Acid†
This work was carried out within the BE-Basic R&D Program, for which an FES subsidy was granted from the Dutch Ministry of Economic Affairs, Agriculture, and Innovation (EL&I).
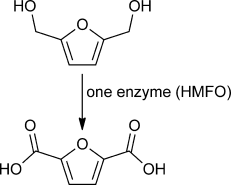
Graphical Abstract
A fourpeat: The recently discovered 5-hydroxymethylfurfural oxidase (HMFO) was found to perform a quadruple oxidation of [5-(hydroxymethyl)furan-2-yl]methanol (see scheme). The biocatalyst can also be used to convert 5-hydroxymethylfurfural into furan-2,5-dicarboxylic acid, thus providing a biobased platform chemical for the production of polymers. The oxidase acts on alcohol groups only and therefore depends on the hydration of aldehyde groups.
Abstract
Furan-2,5-dicarboxylic acid (FDCA) is a biobased platform chemical for the production of polymers. In the past few years, numerous multistep chemical routes have been reported on the synthesis of FDCA by oxidation of 5-hydroxymethylfurfural (HMF). Recently we identified an FAD-dependent enzyme which is active towards HMF and related compounds. This oxidase has the remarkable capability of oxidizing [5-(hydroxymethyl)furan-2-yl]methanol to FDCA, a reaction involving four consecutive oxidations. The oxidase can produce FDCA from HMF with high yield at ambient temperature and pressure. Examination of the underlying mechanism shows that the oxidase acts on alcohol groups only and depends on the hydration of aldehydes for the oxidation reaction required to form FDCA.