Anion-Coordination-Induced Turn-On Fluorescence of an Oligourea-Functionalized Tetraphenylethene in a Wide Concentration Range†
Jie Zhao
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
Search for more papers by this authorDong Yang
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
Search for more papers by this authorDr. Yanxia Zhao
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
Search for more papers by this authorProf. Xiao-Juan Yang
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
Search for more papers by this authorProf. Yao-Yu Wang
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
Search for more papers by this authorCorresponding Author
Prof. Biao Wu
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
State Key Laboratory of Applied Organic Chemistry, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (China)
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)Search for more papers by this authorJie Zhao
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
Search for more papers by this authorDong Yang
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
Search for more papers by this authorDr. Yanxia Zhao
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
Search for more papers by this authorProf. Xiao-Juan Yang
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
Search for more papers by this authorProf. Yao-Yu Wang
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
Search for more papers by this authorCorresponding Author
Prof. Biao Wu
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)
State Key Laboratory of Applied Organic Chemistry, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (China)
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069 (China)Search for more papers by this authorThis work was supported by the National Natural Science Foundation of China (21271149 and 21325102). We thank the referees for their helpful comments.
Graphical Abstract
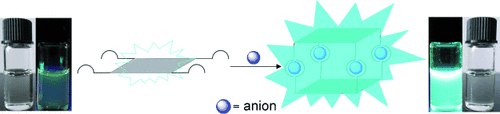
Phosphate ions in a bind: The tetrakis(bisurea)-decorated tetraphenylethene (TPE) displays fluorescence “turn-on” over a wide concentration range upon phosphate coordination. The fluorescence enhancement can be attributed to the restriction of the intramolecular rotation of TPE by anion coordination. This “anion-coordination-induced emission” (ACIE) is another approach for fluorescence turn-on.
Abstract
A tetrakis(bisurea)-decorated tetraphenylethene (TPE) ligand (L2) was designed, which, upon coordination with phosphate ions, displays fluorescence “turn-on” over a wide concentration range, from dilute to concentrated solutions and to the solid state. The fluorescence enhancement can be attributed to the restriction of the intramolecular rotation of TPE by anion coordination. The crystal structure of the A4L2 (A=anion) complex of L2 with monohydrogen phosphate provides direct evidence for the coordination mode of the anion. This “anion-coordination-induced emission” (ACIE) is another approach for fluorescence turn-on in addition to aggregation-induced emission (AIE).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201402169_sm_miscellaneous_information.pdf1.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. W. Thomas III, G. D. Joly, T. M. Swager, Chem. Rev. 2007, 107, 1339–1386;
- 1bY. Hong, J. W. Y. Lam, B. Z. Tang, Chem. Commun. 2009, 4332–4353.
- 2J. B. Birks, Photophysics of Aromatic Molecules, Wiley, London, 1970.
- 3
- 3aJ. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu, B. Z. Tang, Chem. Commun. 2001, 1740–1741;
- 3bB. Z. Tang, X. Zhan, G. Yu, P. P. S. Lee, Y. Liu, D. Zhu, J. Mater. Chem. 2001, 11, 2974–2978.
- 4
- 4a Aggregation-Induced Emission: Applications (Eds.: ), Wiley, Hoboken, 2013;
- 4bY. Hong, J. W. Y. Lam, B. Z. Tang, Chem. Soc. Rev. 2011, 40, 5361–5388;
- 4cJ. Huang, N. Sun, J. Yang, R. Tang, Q. Li, D. Ma, J. Qin, Z. Li, J. Mater. Chem. 2012, 22, 12001–12007;
- 4dC. W. T. Leung, Y. Hong, S. Chen, E. Zhao, J. W. Y. Lam, B. Z. Tang, J. Am. Chem. Soc. 2013, 135, 62–65;
- 4eE. Quartapelle Procopio, M. Mauro, M. Panigati, D. Donghi, P. Mercandelli, A. Sironi, G. D’Alfonso, L. De Cola, J. Am. Chem. Soc. 2010, 132, 14397–14399;
- 4fW. Z. Yuan, P. Lu, S. Chen, J. W. Y. Lam, Z. Wang, Y. Liu, H. S. Kwok, Y. Ma, B. Z. Tang, Adv. Mater. 2010, 22, 2159–2163;
- 4gH. Shi, J. Liu, J. Geng, B. Z. Tang, B. Liu, J. Am. Chem. Soc. 2012, 134, 9569–9572.
- 5
- 5aN. B. Shustova, B. D. McCarthy, M. Dincă, J. Am. Chem. Soc. 2011, 133, 20126–20129;
- 5bT. Noguchi, B. Roy, D. Yoshihara, Y. Tsuchiya, T. Yamamoto, S. Shinkai, Chem. Eur. J. 2014, 20, 381–384.
- 6
- 6aJ. M. Lehn, Acc. Chem. Res. 1978, 11, 49–57;
- 6b Anion Coordination Chemistry, (Eds.: ), Wiley-VCH, Weinheim, 2011;
- 6cP. D. Beer, P. A. Gale, Angew. Chem. 2001, 113, 502–532;
10.1002/1521-3757(20010202)113:3<502::AID-ANGE502>3.0.CO;2-A Google ScholarAngew. Chem. Int. Ed. 2001, 40, 486–516;10.1002/1521-3773(20010202)40:3<486::AID-ANIE486>3.0.CO;2-P CAS PubMed Web of Science® Google Scholar
- 6dE. A. Katayev, Y. A. Ustynyuk, J. L. Sessler, Coord. Chem. Rev. 2006, 250, 3004–3037;
- 6eJ. W. Steed, Chem. Soc. Rev. 2009, 38, 506–519;
- 6fC. Caltagirone, P. A. Gale, Chem. Soc. Rev. 2009, 38, 520–563.
- 7
- 7aF. H. Westheimer, Science 1987, 235, 1173–1178;
- 7bT. Berndt, R. Kumar, Physiology 2009, 24, 17–25.
- 8
- 8aA. I. Jonckheere, J. A. M. Smeitink, R. J. T. Rodenburg, J. Inherited Metab. Dis. 2012, 35, 211–225;
- 8bA. Munshi, R. Ramesh, Genes Cancer 2013, 4, 401–408;
- 8cD. Fisher, L. Krasinska, D. Coudreuse, B. Novák, J. Cell Sci. 2012, 125, 4703–4711.
- 9
- 9aS. J. Brooks, P. A. Gale, M. E. Light, CrystEngComm 2005, 7, 586–591;
- 9bS. J. Brooks, P. A. Gale, M. E. Light, Chem. Commun. 2005, 4696–4698.
- 10
- 10aC. Jia, B. Wu, S. Li, Z. Yang, Q. Zhao, J. Liang, Q.-S. Li, X.-J. Yang, Chem. Commun. 2010, 46, 5376–5378;
- 10bC. Jia, B. Wu, S. Li, X. Huang, X.-J. Yang, Org. Lett. 2010, 12, 5612–5615;
- 10cC. Jia, B. Wu, S. Li, X. Huang, Q. Zhao, Q.-S. Li, X.-J. Yang, Angew. Chem. 2011, 123, 506–510; Angew. Chem. Int. Ed. 2011, 50, 486–490;
- 10dS. Li, C. Jia, B. Wu, Q. Luo, X. Huang, Z. Yang, Q.-S. Li, X.-J. Yang, Angew. Chem. 2011, 123, 5839–5842; Angew. Chem. Int. Ed. 2011, 50, 5721–5724;
- 10eB. Wu, F. Cui, Y. Lei, S. Li, N. D. S. Amadeu, C. Janiak, Y.-J. Lin, L.-H. Weng, Y.-Y. Wang, X.-J. Yang, Angew. Chem. 2013, 125, 5200–5204; Angew. Chem. Int. Ed. 2013, 52, 5096–5100.
- 11
- 11aW. Qin, D. Ding, J. Liu, W. Z. Yuan, Y. Hu, B. Liu, B. Z. Tang, Adv. Funct. Mater. 2012, 22, 771–779;
- 11bL. Liu, G. Zhang, J. Xiang, D. Zhang, D. Zhu, Org. Lett. 2008, 10, 4581–4584;
- 11cT. Noguchi, T. Shiraki, A. Dawn, Y. Tsuchiya, L. T. N. Lien, T. Yamamoto, S. Shinkai, Chem. Commun. 2012, 48, 8090–8092.
- 12Crystal data for complex 1: red-brown block, C280H388K8N32O88P4, M=6046.90, orthorhombic, space group C222(1), a=29.419(6), b=44.908(9), c=28.031(5) Å, V=37033(12) Å3, T=150 K, Z=4, 114503 refl. collected, 31522 unique (Rint=0.0799), R1=0.1172 (I>2σ(I)), wR2=0.3215 (all data). Crystal data for complex 2: yellowish-brown block, C138H177K4N17O39, M=2854.37, monoclinic, space group C2/c, a=44.964(12), b=27.634(7), c=27.188(7) Å, β=91.159(8)°, V=33776(15) Å3, T=150 K, Z=8, 135746 refl. collected, 28561 unique (Rint=0.0948), R1=0.1249 (I>2σ(I)), wR2=0.2987 (all data). CCDC 985431 (1) and 985432 (2) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 13P. S. Ledvina, N. H. Yao, A. Choudhary, F. A. Quiocho, Proc. Natl. Acad. Sci. USA 1996, 93, 6786–6791.