Superconducting Double Perovskite Bismuth Oxide Prepared by a Low-Temperature Hydrothermal Reaction
Mirza H. K. Rubel
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)
Search for more papers by this authorProf. Akira Miura
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)
Search for more papers by this authorProf. Takahiro Takei
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Nobuhiro Kumada
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)Search for more papers by this authorM. Mozahar Ali
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)
Search for more papers by this authorProf. Masanori Nagao
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)
Search for more papers by this authorProf. Satoshi Watauchi
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)
Search for more papers by this authorProf. Isao Tanaka
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)
Search for more papers by this authorProf. Kengo Oka
Materials and Structural laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku Yokohama-city, Kanagawa 226-8503 (Japan)
Search for more papers by this authorProf. Masaki Azuma
Materials and Structural laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku Yokohama-city, Kanagawa 226-8503 (Japan)
Search for more papers by this authorProf. Eisuke Magome
Department of Physical Science, Hiroshima University, 1-3-1 Kagamiyama, Higashihiroshima, Hiroshima 739-8526 (Japan)
Search for more papers by this authorProf. Chikako Moriyoshi
Department of Physical Science, Hiroshima University, 1-3-1 Kagamiyama, Higashihiroshima, Hiroshima 739-8526 (Japan)
Search for more papers by this authorProf. Yoshihiro Kuroiwa
Department of Physical Science, Hiroshima University, 1-3-1 Kagamiyama, Higashihiroshima, Hiroshima 739-8526 (Japan)
Search for more papers by this authorProf. A. K. M. Azharul Islam
Department of Physics, University of Rajshahi, Rajshahi-6205 (Bangladesh)
Search for more papers by this authorMirza H. K. Rubel
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)
Search for more papers by this authorProf. Akira Miura
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)
Search for more papers by this authorProf. Takahiro Takei
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Nobuhiro Kumada
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)Search for more papers by this authorM. Mozahar Ali
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)
Search for more papers by this authorProf. Masanori Nagao
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)
Search for more papers by this authorProf. Satoshi Watauchi
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)
Search for more papers by this authorProf. Isao Tanaka
Center for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae Kofu 400-8511 (Japan)
Search for more papers by this authorProf. Kengo Oka
Materials and Structural laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku Yokohama-city, Kanagawa 226-8503 (Japan)
Search for more papers by this authorProf. Masaki Azuma
Materials and Structural laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku Yokohama-city, Kanagawa 226-8503 (Japan)
Search for more papers by this authorProf. Eisuke Magome
Department of Physical Science, Hiroshima University, 1-3-1 Kagamiyama, Higashihiroshima, Hiroshima 739-8526 (Japan)
Search for more papers by this authorProf. Chikako Moriyoshi
Department of Physical Science, Hiroshima University, 1-3-1 Kagamiyama, Higashihiroshima, Hiroshima 739-8526 (Japan)
Search for more papers by this authorProf. Yoshihiro Kuroiwa
Department of Physical Science, Hiroshima University, 1-3-1 Kagamiyama, Higashihiroshima, Hiroshima 739-8526 (Japan)
Search for more papers by this authorProf. A. K. M. Azharul Islam
Department of Physics, University of Rajshahi, Rajshahi-6205 (Bangladesh)
Search for more papers by this authorGraphical Abstract
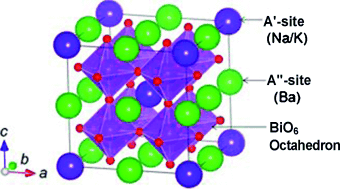
A double helping: The title compound was prepared by hydrothermal reaction of NaBiO3⋅n H2O, Ba(OH)2⋅8 H2O, and KOH at 220 °C. The structure of the double perovskite was determined to be (Na0.25K0.45)(Ba1.00)3(Bi1.00)4O12, and thermally decomposed to a simple perovskite structure above 400 °C. The synthesized compounds exhibit superconductive diamagnetism, and zero resistivity below 8 K.
Abstract
Perovskite-type structures (ABO3) have received significant attention because of their crystallographic aspects and physical properties, but there has been no clear evidence of a superconductor with a double-perovskite-type structure, whose different elements occupy A and/or B sites in ordered ways. In this report, hydrothermal synthesis at 220 °C produced a new superconductor with an A-site-ordered double perovskite structure, (Na0.25K0.45)(Ba1.00)3(Bi1.00)4O12, with a maximum Tc of about 27 K.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201400607_sm_miscellaneous_information.pdf425.8 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1B. A. Baumert, J. Supercond. 1995, 8, 175–181.
- 2A. W. Sleight, J. L. Gillson, P. E. Birstedt, Solid State Commun. 1975, 17, 27–28.
- 3R. J. Cava, B. Batlogg, J. J. Krajewski, R. Farrow, L. W. Rupp, A. E. White, K. Short, W. F. Peck, T. Kometani, Nature 1988, 332, 814–816.
- 4L. F. Mattheiss, D. R. Hamann, Phys. Rev. Lett. 1988, 60, 2681–2684.
- 5R. J. Cava, B. Batlogg, G. P. Espinosa, A. P. Ramirez, J. Krajewskij, W. F. Peck, Jr., W. Ruppl, Jr., S. Cooper, Nature 1989, 339, 291–293.
- 6D. Jung, E.-K. Choi, Bull. Korean Chem. Soc. 1999, 20, 1045–1048.
- 7V. Meregalli, S. Y. Savrasov, Phys. Rev. B 1998, 57, 14453–14469.
- 8M. Shirai, N. Suzuki, K. Motizuki, J. Phys. Condens. Matter 1990, 2, 3553.
- 9H. Jiang, N. Kumada, Y. Yonesaki, T. Takei, N. Kinomura, M. Yashima, M. Azuma, K. Oka, Y. Shimakawa, Jpn. J. Appl. Phys. 2009, 48, 010216–010218.
- 10G. Zhang, G. Li, F. Huang, F. Liao, K. Li, Y. Wang, J. Lin, J. Alloys Compd. 2011, 509, 9804–9808.
- 11Y. Mizuguchi, S. Demura, K. Deguchi, Y. Takano, H. Fujihisa, Y. Gotoh, H. Izawa, O. Miura, J. Phys. Soc. Jpn. 2012, 81, 114725–114729.
- 12E. A. Ekimov, V. A. Sidorov, E. D. Bauer, N. N. Mel′nik, N. J. Curro, J. D. Thompson, S. M. Stishov, Nature 2004, 428, 542–545.
- 13Y. Takano, M. Nagao, I. Sakaguchi, M. Tachiki, T. Hatano, K. Kobayashi, H. Umezawa, H. Kawarada, Appl. Phys. Lett. 2004, 85, 2851–2853.
- 14S. Yamanaka, K. Hotehama, H. Kawaji, Nature 1998, 392, 580–582.
- 15H. Kawaji, K. Hotehama, S. Yamanaka, Chem. Mater. 1997, 9, 2127–2130.
- 16T. Ito, Y. Fudamoto, A. Fukaya, I. M. Gat-Malureanu, M. I. Larkin, P. L. Russo, A. Savici, Y. J. Uemura, K. Groves, R. Breslow, K. Hotehama, S. Yamanaka, P. Kyriakou, M. Rovers, G. M. Luke, K. M. Kojima, Phys. Rev. B 2004, 69, 134522–134534.
- 17K. Hyun-Tak, K. Kwang-Yong, K. Won-Jeong, J. Korean Phys. Soc. 2001, 39, 1013–1018.
- 18Y. Idemoto, Y. Iwata, K. Fueki, Phys. C 1994, 222, 257–266.
- 19Y. Idemoto, Y. Iwata, K. Fueki, Phys. C 1992, 201, 43–49.
- 20R. D. Shannon, Acta Crystallogr. Sect. A 1976, 32, 751–767.
- 21B. E. Conway, Ionic hydration in chemistry and biophysics, Elsevier Publ. Co., Amsterdam, 1981, pp. 312.
- 22H. Jiang, N. Kumada, Y. Yonesaki, T. Takei, N. Kinomura, J. Ceram. Soc. Jpn. 2009, 117, 214–216.
- 23D. G. Hinks, B. Dabrowski, J. D. Jorgensen, A. W. Mitchell, D. R. Richards, S. Pei, D. Shi, Nature 1988, 333, 836–838.
- 24F. Izumi, K. Momma, Solid State Phenom. 2007, 130, 15–20.
- 25K. Momma, F. Izumi, J. Appl. Crystallogr. 2008, 41, 653–658.
- 26S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. I. J. Probert, K. Refson, M. C. Payne, Z. Kristallogr. 2005, 220, 567–570.
- 27J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 1996, 77, 3865–3868.