Metal-Free Hydrogenation of Unsaturated Hydrocarbons Employing Molecular Hydrogen
Corresponding Author
Priv.-Doz. Dr. Jan Paradies
Institute for Organic Chemistry, Karlsruhe Institute of Technology (KIT), Fritz-Haber-Weg 6, 76131 Karlsruhe (Germany) http://www.paradies-group.de
Institute for Organic Chemistry, Karlsruhe Institute of Technology (KIT), Fritz-Haber-Weg 6, 76131 Karlsruhe (Germany) http://www.paradies-group.deSearch for more papers by this authorCorresponding Author
Priv.-Doz. Dr. Jan Paradies
Institute for Organic Chemistry, Karlsruhe Institute of Technology (KIT), Fritz-Haber-Weg 6, 76131 Karlsruhe (Germany) http://www.paradies-group.de
Institute for Organic Chemistry, Karlsruhe Institute of Technology (KIT), Fritz-Haber-Weg 6, 76131 Karlsruhe (Germany) http://www.paradies-group.deSearch for more papers by this authorGraphical Abstract
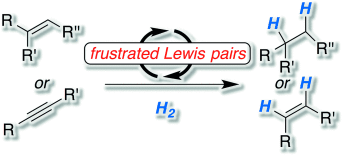
Recent metal-free approaches to the hydrogenation of nonpolar double and triple bonds using molecular hydrogen are described. Despite transition-metal-based methodologies for these fundamental chemical transformations, metal-free alternatives are highly desirable. Such technology has only been recently introduced with the aid of frustrated Lewis pairs.
Abstract
The metal-free activation of hydrogen by frustrated Lewis pairs (FLPs) is a valuable method for the hydrogenation of polarized unsaturated molecules ranging from imines, enamines, and silyl enol ethers to heterocycles. However, one of the most important applications of hydrogenation technology is the conversion of unsaturated hydrocarbons into alkanes or alkenes. Despite the fast development of the FLP chemistry, such reactions proved as highly challenging. This Minireview provides an overview of the basic concepts of FLP chemistry, the challenge in the hydrogenation of unsaturated hydrocarbons, and first solutions to this central transformation.
References
- 1“Hydrogenation and Dehydrogenation”: P. N. Rylander in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
- 2
- 2aS. Shima, E. J. Lyon, R. K. Thauer, B. Mienert, E. Bill, J. Am. Chem. Soc. 2005, 127, 10430–10435;
- 2bO. Pilak, B. Mamat, S. Vogt, C. H. Hagemeier, R. K. Thauer, S. Shima, C. Vonrhein, E. Warkentin, U. Ermler, J. Mol. Biol. 2006, 358, 798–809;
- 2cA. P. Scott, B. T. Golding, L. Radom, New J. Chem. 1998, 22, 1171–1173;
- 2dA. Berkessel, R. K. Thauer, Angew. Chem. 1995, 107, 2418–2421;
10.1002/ange.19951072011 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 2247–2250;
- 2eJ. H. Teles, S. Brode, A. Berkessel, J. Am. Chem. Soc. 1998, 120, 1345–1346.
- 3
- 3aG. H. Spikes, J. C. Fettinger, P. P. Power, J. Am. Chem. Soc. 2005, 127, 12232–12233;
- 3bA. Berkessel, T. J. S. Schubert, T. N. Muller, J. Am. Chem. Soc. 2002, 124, 8693–8698;
- 3cC. Walling, L. Bollyky, J. Am. Chem. Soc. 1964, 86, 3750–3752.
- 4G. C. Welch, R. R. S. Juan, J. D. Masuda, D. W. Stephan, Science 2006, 314, 1124–1126.
- 5H. C. Brown, H. I. Schlesinger, S. Z. Cardon, J. Am. Chem. Soc. 1942, 64, 325–333.
- 6
- 6aD. W. Stephan, Org. Biomol. Chem. 2008, 6, 1535–1539;
- 6bD. W. Stephan, Dalton Trans. 2009, 3129–3136.
- 7P. A. Chase, G. C. Welch, T. Jurca, D. W. Stephan, Angew. Chem. 2007, 119, 8196–8199;
10.1002/ange.200702908 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8050–8053.
- 8G. C. Welch, D. W. Stephan, J. Am. Chem. Soc. 2007, 129, 1880–1881.
- 9
- 9aD. W. Stephan, G. Erker, Angew. Chem. 2010, 122, 50–81; Angew. Chem. Int. Ed. 2010, 49, 46–76;
- 9bG. Erker, Pure Appl. Chem. 2012, 84, 2203–2217.
- 10
- 10aC. M. Mömming, E. Otten, G. Kehr, R. Fröhlich, S. Grimme, D. W. Stephan, G. Erker, Angew. Chem. 2009, 121, 6770–6773;
10.1002/ange.200901636 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6643–6646;
- 10bI. Peuser, R. C. Neu, X. X. Zhao, M. Ulrich, B. Schirmer, J. A. Tannert, G. Kehr, R. Fröhlich, S. Grimme, G. Erker, D. W. Stephan, Chem. Eur. J. 2011, 17, 9640–9650;
- 10cX. X. Zhao, D. W. Stephan, Chem. Commun. 2011, 47, 1833–1835;
- 10dM. Harhausen, R. Fröhlich, G. Kehr, G. Erker, Organometallics 2012, 31, 2801–2809;
- 10eM. Sajid, A. Stute, A. J. P. Cardenas, B. J. Culotta, J. A. M. Hepperle, T. H. Warren, B. Schirmer, S. Grimme, A. Studer, C. G. Daniliuc, R. Fröhlich, J. L. Petersen, G. Kehr, G. Erker, J. Am. Chem. Soc. 2012, 134, 10156–10168.
- 11M. Sajid, A. Klose, B. Birkmann, L. Y. Liang, B. Schirmer, T. Wiegand, H. Eckert, A. J. Lough, R. Fröhlich, C. G. Daniliuc, S. Grimme, D. W. Stephan, G. Kehr, G. Erker, Chem. Sci. 2013, 4, 213–219.
- 12A. J. P. Cardenas, B. J. Culotta, T. H. Warren, S. Grimme, A. Stute, R. Fröhlich, G. Kehr, G. Erker, Angew. Chem. 2011, 123, 7709–7713;
10.1002/ange.201101622 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 7567–7571.
- 13
- 13aE. Otten, R. C. Neu, D. W. Stephan, J. Am. Chem. Soc. 2009, 131, 9918–9919;
- 13bR. C. Neu, E. Otten, A. Lough, D. W. Stephan, Chem. Sci. 2011, 2, 170–176.
- 14D. W. Stephan, Org. Biomol. Chem. 2012, 10, 5740–5746.
- 15
- 15aT. Soós, Pure Appl. Chem. 2011, 83, 667–675;
- 15bD. W. Stephan, S. Greenberg, T. W. Graham, P. Chase, J. J. Hastie, S. J. Geier, J. M. Farrell, C. C. Brown, Z. M. Heiden, G. C. Welch, M. Ullrich, Inorg. Chem. 2011, 50, 12338–12348;
- 15cJ. Paradies, Synlett 2013, 24, 777–780.
- 16
- 16aA. Hamza, A. Stirling, T. A. Rokob, I. Papai, Int. J. Quantum Chem. 2009, 109, 2416–2425;
- 16bT. A. Rokob, I. Bako, A. Stirling, A. Hamza, I. Papai, J. Am. Chem. Soc. 2013, 135, 4425–4437;
- 16cT. A. Rokob, A. Hamza, I. Papai, J. Am. Chem. Soc. 2009, 131, 10701–10710;
- 16dS. Grimme, H. Kruse, L. Goerigk, G. Erker, Angew. Chem. 2010, 122, 1444–1447; Angew. Chem. Int. Ed. 2010, 49, 1402–1405;
- 16eB. Schirmer, S. Grimme, Chem. Commun. 2010, 46, 7942–7944.
- 17For an extensive study on the H2 activation by six different FLPs, see Ref. [16b].
- 18
- 18aV. Sumerin, F. Schulz, M. Nieger, M. Leskela, T. Repo, B. Rieger, Angew. Chem. 2008, 120, 6090–6092;
10.1002/ange.200800935 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6001–6003;
- 18bV. Sumerin, K. Chernichenko, M. Nieger, M. Leskela, B. Rieger, T. Repo, Adv. Synth. Catal. 2011, 353, 2093–2110;
- 18cP. A. Chase, T. Jurca, D. W. Stephan, Chem. Commun. 2008, 1701–1703;
- 18dS. J. Geier, P. A. Chase, D. W. Stephan, Chem. Commun. 2010, 46, 4884–4886;
- 18eT. Mahdi, Z. M. Heiden, S. Grimme, D. W. Stephan, J. Am. Chem. Soc. 2012, 134, 4088–4091.
- 19
- 19aP. A. Chase, D. W. Stephan, Angew. Chem. 2008, 120, 7543–7547;
10.1002/ange.200802596 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7433–7437;
- 19bD. Holschumacher, T. Bannenberg, C. G. Hrib, P. G. Jones, M. Tamm, Angew. Chem. 2008, 120, 7538–7542;
10.1002/ange.200802705 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7428–7432;
- 19cS. Kronig, E. Theuergarten, D. Holschumacher, T. Bannenberg, C. G. Daniliuc, P. G. Jones, M. Tamm, Inorg. Chem. 2011, 50, 7344–7359.
- 20
- 20aJ. M. Farrell, J. A. Hatnean, D. W. Stephan, J. Am. Chem. Soc. 2012, 134, 15728–15731;
- 20bM. A. Dureen, A. Lough, T. M. Gilbert, D. W. Stephan, Chem. Commun. 2008, 4303–4305;
- 20cE. R. Clark, A. Del Grosso, M. J. Ingleson, Chem. Eur. J. 2013, 19, 2462–2466.
- 21
- 21aY. T. Zhang, G. M. Miyake, E. Y. X. Chen, Angew. Chem. 2010, 122, 10356–10360; Angew. Chem. Int. Ed. 2010, 49, 10158–10162;
- 21bG. Ménard, L. Tran, D. W. Stephan, Dalton Trans. 2013, 42, 13685–13691;
- 21cG. Ménard, D. W. Stephan, Angew. Chem. 2012, 124, 8397–8400;
10.1002/ange.201203362 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 8272–8275;
- 21dS. Roters, C. Appelt, H. Westenberg, A. Hepp, J. C. Slootweg, K. Lammertsma, W. Uhl, Dalton Trans. 2012, 41, 9033–9045.
- 22G. Eros, H. Mehdi, I. Papai, T. A. Rokob, P. Kiraly, G. Tarkanyi, T. Soós, Angew. Chem. 2010, 122, 6709–6713;
10.1002/ange.201001518 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 6559–6563.
- 23
- 23aD. J. Chen, Y. T. Wang, J. Klankermayer, Angew. Chem. 2010, 122, 9665–9668; Angew. Chem. Int. Ed. 2010, 49, 9475–9478;
- 23bD. J. Chen, J. Klankermayer, Chem. Commun. 2008, 2130–2131;
- 23cG. Ghattas, D. J. Chen, F. F. Pan, J. Klankermayer, Dalton Trans. 2012, 41, 9026–9028;
- 23dY. B. Liu, H. F. Du, J. Am. Chem. Soc. 2013, 135, 6810–6813.
- 24
- 24aG. Eros, K. Nagy, H. Mehdi, I. Papai, P. Nagy, P. Kiraly, G. Tarkanyi, T. Soós, Chem. Eur. J. 2012, 18, 574–585;
- 24bT. Mahdi, J. N. del Castillo, D. W. Stephan, Organometallics 2013, 32, 1971–1978.
- 25
- 25aL. Greb, P. Oña-Burgos, A. Kubas, F. C. Falk, F. Breher, K. Fink, J. Paradies, Dalton Trans. 2012, 41, 9056–9060;
- 25bH. D. Wang, R. Fröhlich, G. Kehr, G. Erker, Chem. Commun. 2008, 5966–5968.
- 26
- 26aP. Spies, S. Schwendemann, S. Lange, G. Kehr, R. Fröhlich, G. Erker, Angew. Chem. 2008, 120, 7654–7657;
10.1002/ange.200801432 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7543–7546;
- 26bS. Schwendemann, R. Fröhlich, G. Kehr, G. Erker, Chem. Sci. 2011, 2, 1842–1849.
- 27P. Spies, G. Erker, G. Kehr, K. Bergander, R. Fröhlich, S. Grimme, D. W. Stephan, Chem. Commun. 2007, 5072–5074.
- 28aB. Inés, D. Palomas, S. Holle, S. Steinberg, J. A. Nicasio, M. Alcarazo, Angew. Chem. 2012, 124, 12533–12536;
10.1002/ange.201205348 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 12367–12369;
- 28bL. Greb, C. G. Daniliuc, K. Bergander, J. Paradies, Angew. Chem. 2013, 125, 5989–5992;
10.1002/ange.201210175 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 5876–5879;
- 28cJ. A. Nicasio, S. Steinberg, B. Ines, M. Alcarazo, Chem. Eur. J. 2013, 19, 11016–11020.
- 29
- 29aL. Greb, P. Oña-Burgos, B. Schirmer, S. Grimme, D. W. Stephan, J. Paradies, Angew. Chem. 2012, 124, 10311–10315;
10.1002/ange.201204007 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 10164–10168;
- 29bL. Greb, S. Tussing, B. Schirmer, P. Oña-Burgos, K. Kaupmees, M. Lokov, I. Leito, S. Grimme, J. Paradies, Chem. Sci. 2013, 4, 2788–2796.
- 30L. J. Hounjet, C. Bannwarth, C. N. Garon, C. B. Caputo, S. Grimme, D. W. Stephan, Angew. Chem. 2013, 125, 7640–7643;
10.1002/ange.201303166 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 7492–7495.
- 31Y. Segawa, D. W. Stephan, Chem. Commun. 2012, 48, 11963–11965.
- 32
- 32aF. L. Ramp, E. J. Dewitt, L. E. Trapasso, J. Org. Chem. 1962, 27, 4368–4372;
- 32bE. J. Dewitt, L. E. Trapasso, F. L. Ramp, J. Am. Chem. Soc. 1961, 83, 4672.
- 33A. Stute, G. Kehr, R. Fröhlich, G. Erker, Chem. Commun. 2011, 47, 4288–4290.
- 34D. J. Chen, V. Leich, F. F. Pan, J. Klankermayer, Chem. Eur. J. 2012, 18, 5184–5187.
- 35
- 35aC. Fan, L. G. Mercier, W. E. Piers, H. M. Tuononen, M. Parvez, J. Am. Chem. Soc. 2010, 132, 9604–9606;
- 35bA. Y. Houghton, V. A. Karttunen, C. Fan, W. E. Piers, H. M. Tuononen, J. Am. Chem. Soc. 2013, 135, 941–947.
- 36
- 36aZ. Lu, Z. Cheng, Z. Chen, L. Weng, Z. H. Li, H. Wang, Angew. Chem. 2011, 123, 12435; Angew. Chem. Int. Ed. 2011, 50, 12227;
- 36bG. I. Nikonov, S. F. Vyboishchikov, O. G. Shirobokov, J. Am. Chem. Soc. 2012, 134, 5488–5491.
- 37D. J. Parks, W. E. Piers, G. P. A. Yap, Organometallics 1998, 17, 5492–5503.
- 38Y. Wang, W. Chen, Z. Lu, Z. H. Li, H. Wang, Angew. Chem. 2013, 125, 7644–7647; Angew. Chem. Int. Ed. 2013, 52, 7496–7499.
- 39K. Chernichenko, A. Madarasz, I. Papai, M. Nieger, M. Leskela, T. Repo, Nat. Chem. 2013, 5, 718–723.
- 40K. Chernichenko, M. Nieger, M. Leskela, T. Repo, Dalton Trans. 2012, 41, 9029–9032.
- 41
- 41aC. Chen, F. Eweiner, B. Wibbeling, R. Fröhlich, S. Senda, Y. Ohki, K. Tatsumi, S. Grimme, G. Kehr, G. Erker, Chem. Asian J. 2010, 5, 2199–2208;
- 41bC. Rosorius, G. Kehr, R. Fröhlich, S. Grimme, G. Erker, Organometallics 2011, 30, 4211–4219.